Hydro-thermal synthesis process of phosphide
A technology of hydrothermal synthesis and phosphide, applied in the direction of phosphide, phosphorus compound, inorganic chemistry, etc., can solve the problems of danger, personal and environmental damage in the heat treatment process of white phosphorus, and achieve the goal of increasing yield, avoiding pollution and reducing production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Weigh 4.863g NiCl 2 ·6H 2 O. 6.335g of red phosphorus is fully ground by adding 5mL of deionized water; then place it in a 75mL lined stainless steel hydrothermal kettle, add deionized water until the filling volume is 80% of the liner volume, and tighten.
[0021] (2) Heat the hydrothermal kettle to 200°C and keep it warm for 24 hours; then cool it down to room temperature naturally.
[0022] (3) Open the hydrothermal kettle, filter the obtained solution with suction, and then wash several times with deionized water and absolute ethanol respectively.
[0023] (4) Put the obtained powder in a drying oven, and dry at 40° C. to obtain Ni2P powder.
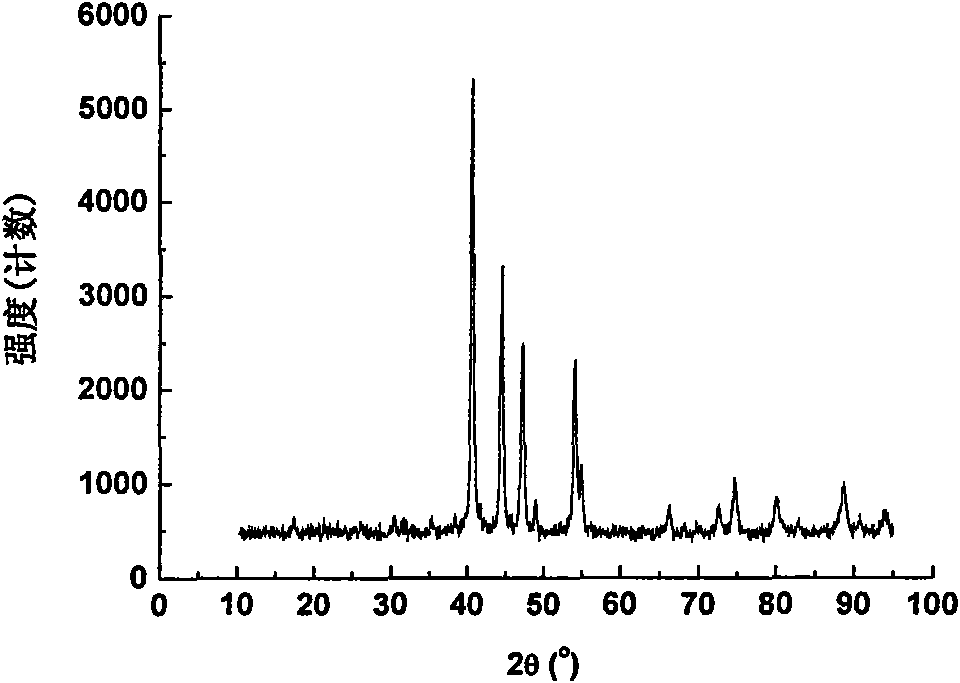
[0024] Ni prepared by this embodiment 2 The phase of P powder is as figure 1 As shown, it can be seen from the figure that the obtained Ni 2 P powder is pure in phase.
Embodiment 2
[0026] (1) Weigh 4.863g NiCl 2 ·6H 2 O. 6.335g of red phosphorus is fully ground by adding 5mL of deionized water; then place it in a 75mL lined stainless steel hydrothermal kettle, add deionized water until the filling volume is 80% of the liner volume, and tighten.
[0027] (2) Heat the hydrothermal kettle to 200° C. and keep it warm for 48 hours; then cool down naturally to room temperature.
[0028] (3) Open the hydrothermal kettle, filter the obtained solution with suction, and then wash several times with deionized water and absolute ethanol respectively.
[0029] (4) Place the obtained powder in a drying oven and dry at 40°C to obtain Ni 12 P 5 Powder.
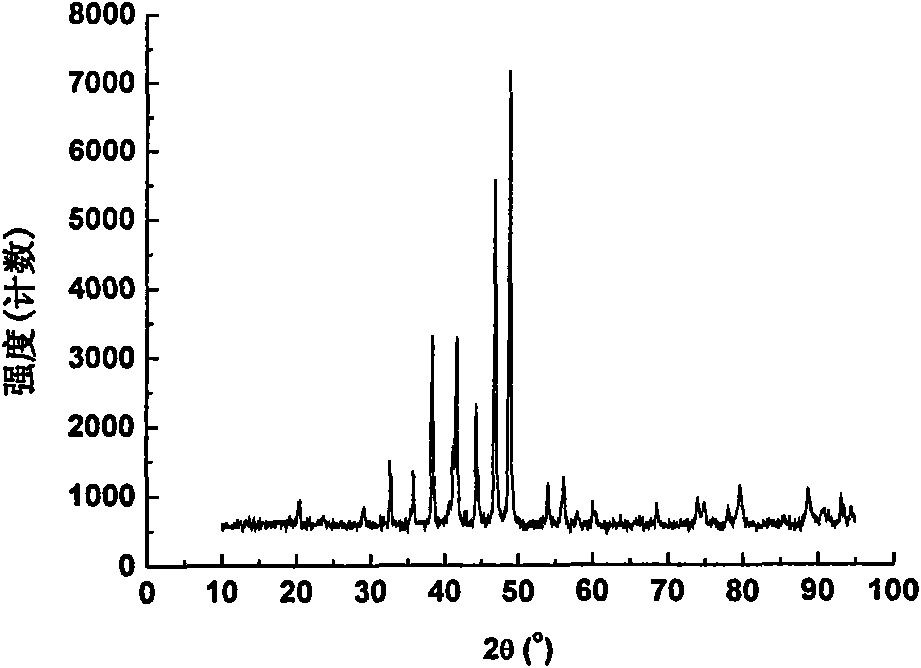
[0030] Ni prepared by this embodiment 12 P 5 The phase of the powder is like figure 2 As shown, it can be seen from the figure that the obtained Ni 12 P 5 The powder is pure in phase.
Embodiment 3
[0032] (1) Weigh 1.439g ultra-fine copper powder, 6.925g red phosphorus and add 5mL deionized water to fully grind; then place it in an 80mL lined stainless steel hydrothermal kettle, add deionized water until the filling volume is the liner volume 80% tightened.
[0033] (2) Heat the hydrothermal kettle to 200°C and keep it warm for 24 hours; then cool it down to room temperature naturally.
[0034] (3) Open the hydrothermal kettle, filter the obtained solution with suction, and then wash several times with deionized water and absolute ethanol respectively.
[0035] (4) Place the obtained powder in a drying oven and dry at 40°C to obtain Cu 3 P powder.
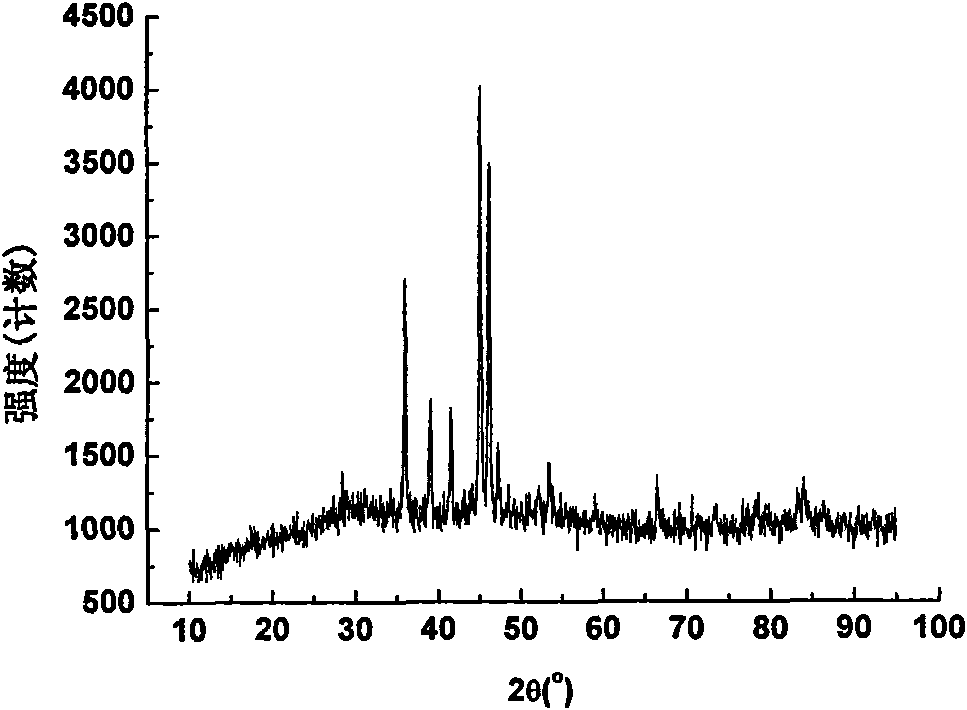
[0036] Cu prepared by this embodiment 3 The phase of P powder is as image 3 As shown, it can be seen from the figure that the obtained Cu 3 P powder is pure in phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com