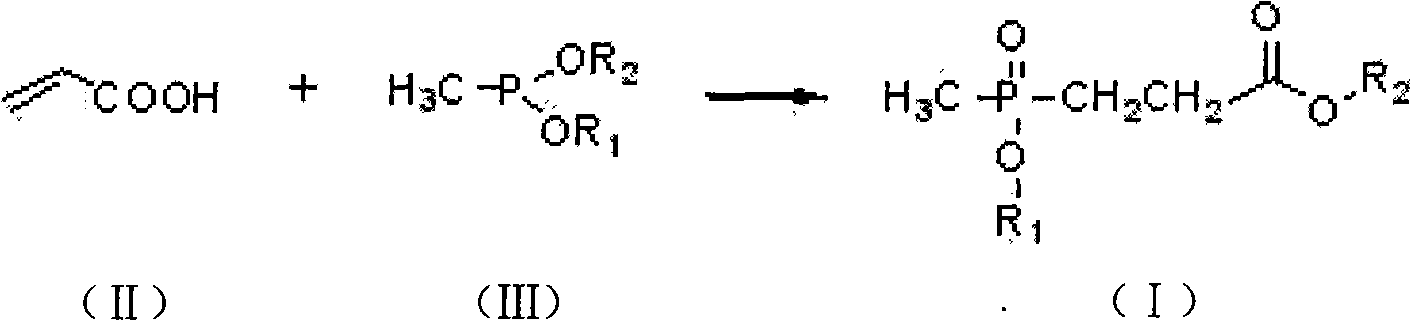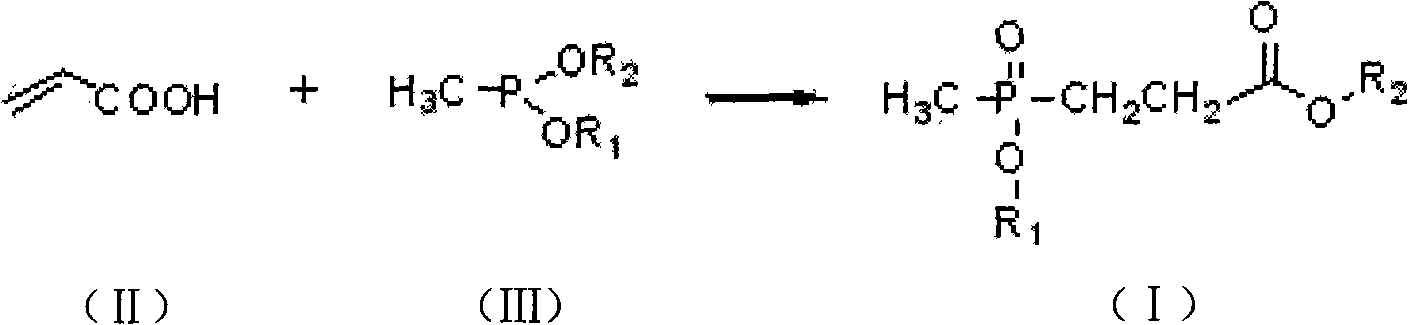Preparation method of 3-(methyl alkoxy phosphoryl) propionic acid ester compound
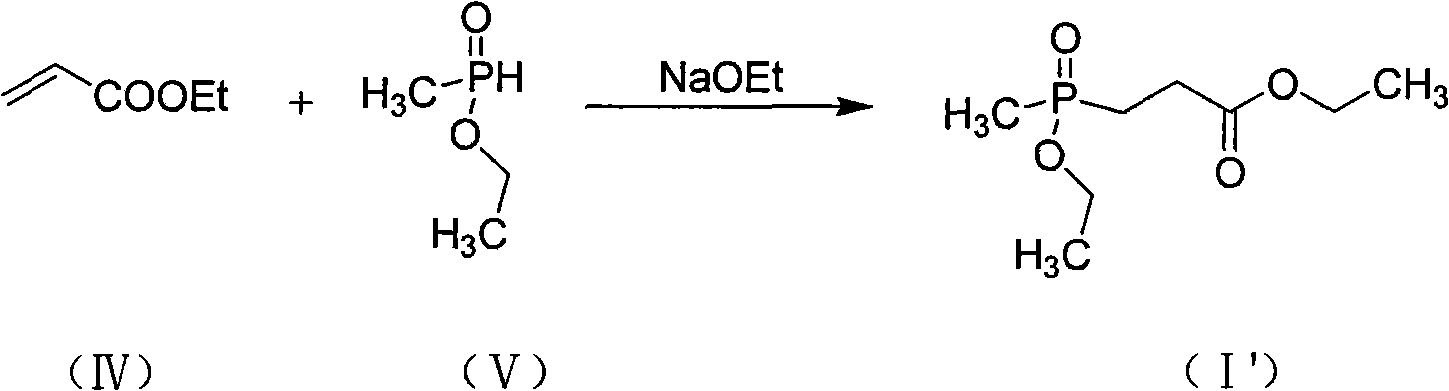
A technology of methyl alkoxyphosphoryl and methyl dialkyl phosphite, which is applied in the field of preparation of 3-propionate compounds, can solve the problems of cumbersome operation, large amount of three wastes, and unfavorable industrial production, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Under nitrogen protection, in a 500ml four-neck round-bottomed flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel, add 204 grams (1.5mol) of diethyl methyl phosphite, and then add the constant pressure drop Add 110.6 grams (1.515mol) of 99% acrylic acid in the funnel; cool down to 5°C, start dropwise reaction, temperature control is at 10°C, dropwise addition is completed in 2 hours, then insulated and stirred for 4 hours, and the reaction ends to obtain 3- (Methylethoxyphosphoryl) ethyl propionate product 288.0 grams, content 96.5%, yield 96.0%.
[0016] Structure detection data ( 1 HNMR (600MHz, CDCl 3 ): 84.16 (q, 2, J=7.3Hz, COOCH 2 CH 3 ) 4.07 (q, 2, J=7.3Hz, POCH 2 CH 3 ), 2.65-2.58 (m, 2, PCH 2 CH 2 COO) 2.09-2.04 (m, 2, PCH 2 CH2COO), 1.49(d, 3, J=13.7Hz, PCH3), 1.33(t, 3, J=7.0Hz, COOCH2CH3), 1.27(t, 3, J=7.1Hz, POCH2CH3);
[0017] 13 CNMR (600MHz, CDCl 3 ): δ172.06, 60.84, 60.10, 26.94, 24.80, 16.50, 14.07...
Embodiment 2
[0019] Under nitrogen protection, in a 500ml four-neck round-bottomed flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel, add 162 grams (1.5mol) of dimethyl methylphosphite, and then add the constant pressure drop Add 110.3 grams (1.515mol) of 99% acrylic acid in the funnel; cool down to 5°C, start dropwise reaction, temperature control is at 0°C, dropwise addition is completed in 3 hours, then insulated and stirred for 5 hours, and the reaction ends to obtain 3- (Methylmethoxyphosphoryl) methyl propionate product 250.4 grams, content is 96.3%, yield 95.7%.
[0020] Structure detection data ( 1 HNMR (600MHz, CDCl 3 ): δ3.81 (d, 3,, COOCH 3 ), 3.53 (m, 3, POCH 3 ), 2.63-2.57 (m, 2, PCH 2 CH 2 COO), 2.07-2.01 (m, 2, PCH 2 CH 2 COO), 1.49 (d, 3, J=13.7Hz, PCH 3 );
[0021] 13 CNMR (600MHz, CDCl 3 ): δ172.7, 58.80, 58.10, 26.2, 24.30, 14.7).
Embodiment 3
[0023] Under nitrogen protection, in a 500ml four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel, add 162 grams (1.5mol) of methyl dimethyl phosphite and 1000ml of toluene, and then add Add 110.3 grams (1.515 mol) of 99% acrylic acid into the pressure drop funnel; cool down to 5°C, start the dropwise reaction, control the temperature at 0°C, and complete the dropwise addition in 2.5 hours, then keep warm and stir for 8 hours, and the reaction ends. 250.1 g of methyl 3-(methylmethoxyphosphoryl)propionate were obtained, with a content of 96.6% and a yield of 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com