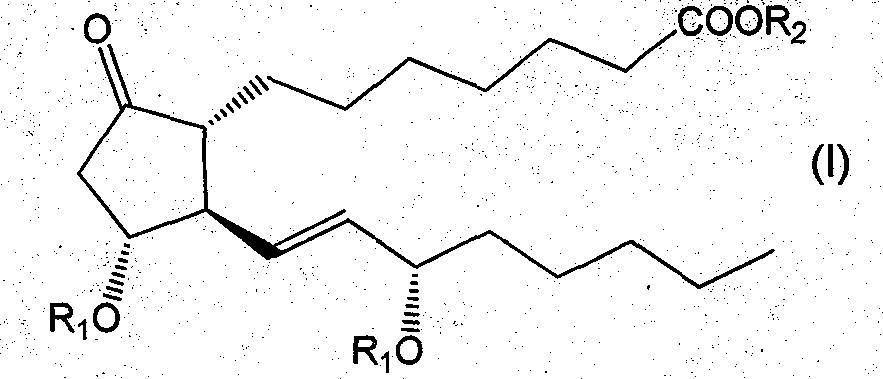
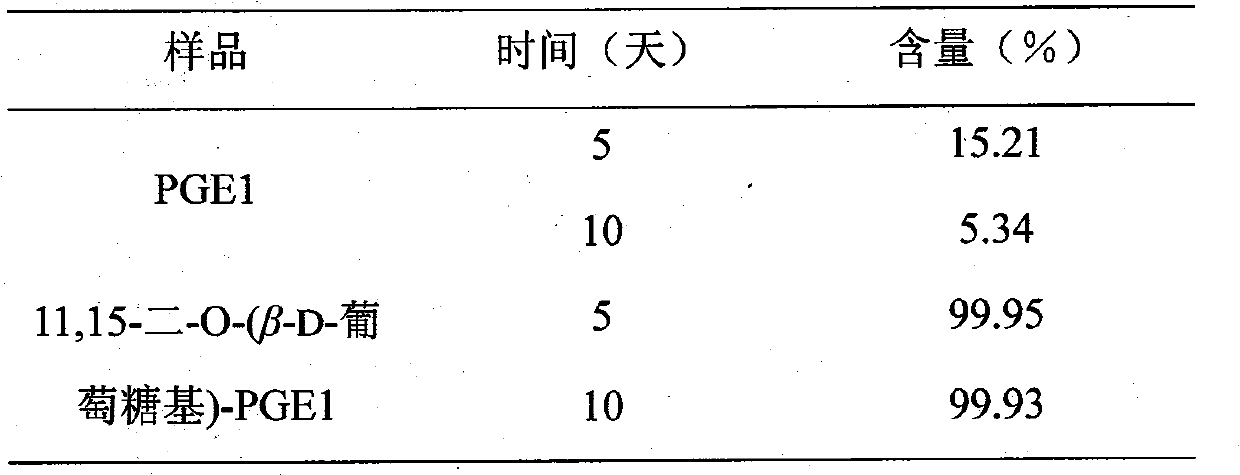
Glycoside derivatives of prostaglandin e1 and preparation method thereof
A prostaglandin and derivative technology, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of short half-life, rapid metabolic inactivation, poor water solubility of prostaglandin E1, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
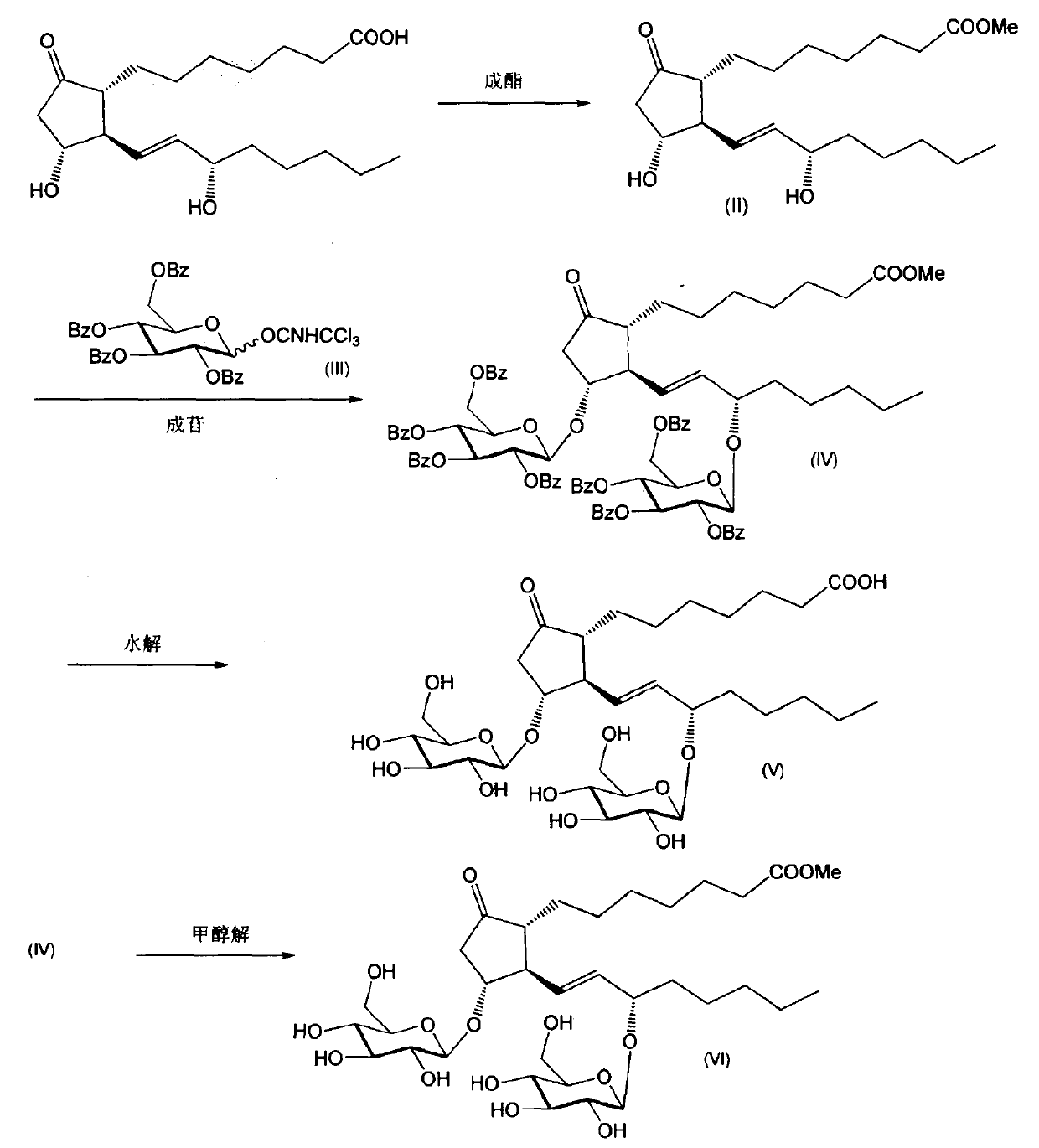
[0023] Synthesis of 11,15-di-O-(2,3,4,6,-tetra-O-benzoyl-β-D-glucosyl)-PGE1 methyl ester (compound (IV))
[0024] (1) Take 0.50g (1.4mmol) PGE1, 4.7ml (0.12mol) methanol, 0.4ml (2.9mmol) triethylamine, add 10ml dichloromethane to dissolve. in N 2 After stirring for 5 minutes, 0.30 g (1.6 mmol) of p-toluenesulfonyl chloride was added. Stir at room temperature for 2.5 hours, wash with saturated ammonium chloride solution three times, and concentrate the organic phase to dryness. The residue was added to 50 ml of TRIS buffer solution of pH 8 and stirred for 15 minutes, then extracted with diethyl ether, the organic phase was washed with water until neutral, dried by adding anhydrous sodium sulfate, filtered, and concentrated. Pressurized column chromatography (chloroform-methanol=98:2) gave 0.24 g of PGE1 methyl ester as a white solid. ESI MS m / z: 391.4 (M+Na) + . 1 H NMR (CDCl 3 , 300MHz): δ0.89(t, 3H, J=6.9Hz), 1.12-1.83(m, 18H), 1.85-2.66(m, 7H), 2.73(dd, 1H, J=18.0, 7.6...
Embodiment 2
[0027] Synthesis of 11,15-di-O-(β-D-glucosyl)-PGE1 (compound (V))
[0028] Take 0.80g (0.52mmol) of 11,15-di-O-(2,3,4,6,-tetra-O-benzoyl-β-D-glucosyl)-PGE1 methyl ester obtained in Example 1, 0.21g (5.2mmol) sodium hydroxide, add 3ml dichloromethane, 10ml methanol, 2ml water to dissolve, stir at room temperature for 4 hours, add strong acidic cation exchange resin to neutralize to neutral, filter, concentrate the filtrate, wash with ether The residue. The residue was subjected to pressure column chromatography (chloroform-methanol-water=7:3:1) to obtain 0.31 g of the title compound. ESIMS m / z: 701.8 (M+Na) + . 1 H NMR (pyridine-d 5 , 300MHz): δ0.89(t, 3H, J=6.9Hz), 1.13-1.84(m, 18H), 1.87-2.67(m, 5H), 2.74(dd, 1H, J=18.0, 7.6Hz), 3.90-4.00(m, 2H), 4.03-4.07(m, 4H), 4.24-4.26(m, 4H), 4.39-4.45(m, 2H), 4.93(m, 2H), 5.63(m, 2H), 11.04(s, 1H).
Embodiment 3
[0030] Synthesis of 11,15-di-O-(β-D-glucosyl)-PGE1 methyl ester (compound (VI))
[0031] Take 0.80g (0.52mmol) of 11,15-di-O-(2,3,4,6,-tetra-O-benzoyl-β-D-glucosyl)-PGE1 methyl ester obtained in Example 1, Add 6ml of dichloromethane, dissolve in 6ml of methanol, add 0.52ml (0.52mmol) of 1M sodium methoxide methanol solution, stir at room temperature for 4 hours, add strong acid cation exchange resin to neutralize to neutral, filter, concentrate the filtrate, and diethyl ether Wash residue. The residue was subjected to pressure column chromatography (chloroform-methanol-water=7:3:1) to obtain 0.32 g of the title compound. ESI MS m / z: 715.2 (M+Na) + . 1 H NMR (pyridine-d 5 , 300MHz): δ0.89(t, 3H, J=6.9Hz), 1.13-1.84(m, 18H), 1.87-2.67(m, 5H), 2.74(dd, 1H, J=18.0, 7.6Hz), 3.68(s, 3H), 3.90-4.00(m, 2H), 4.03-4.07(m, 4H), 4.24-4.26(m, 4H), 4.39-4.45(m, 2H), 4.93(m, 2H), 5.63 (m, 2H), 11.04 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com