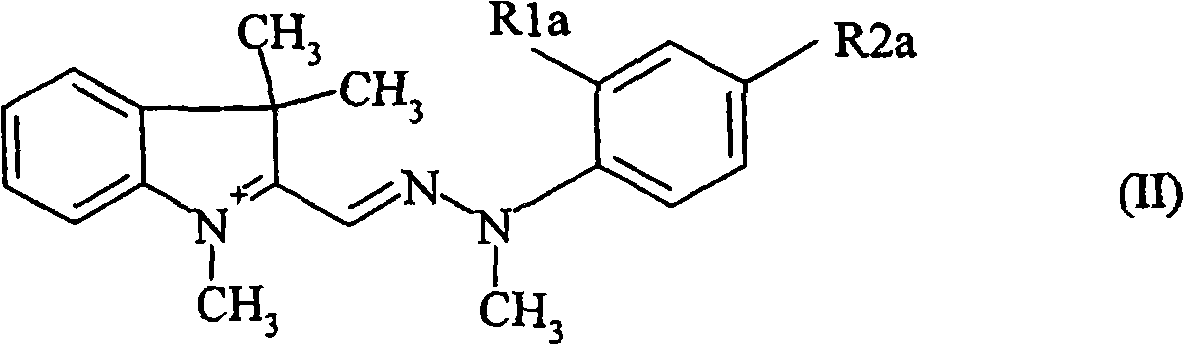
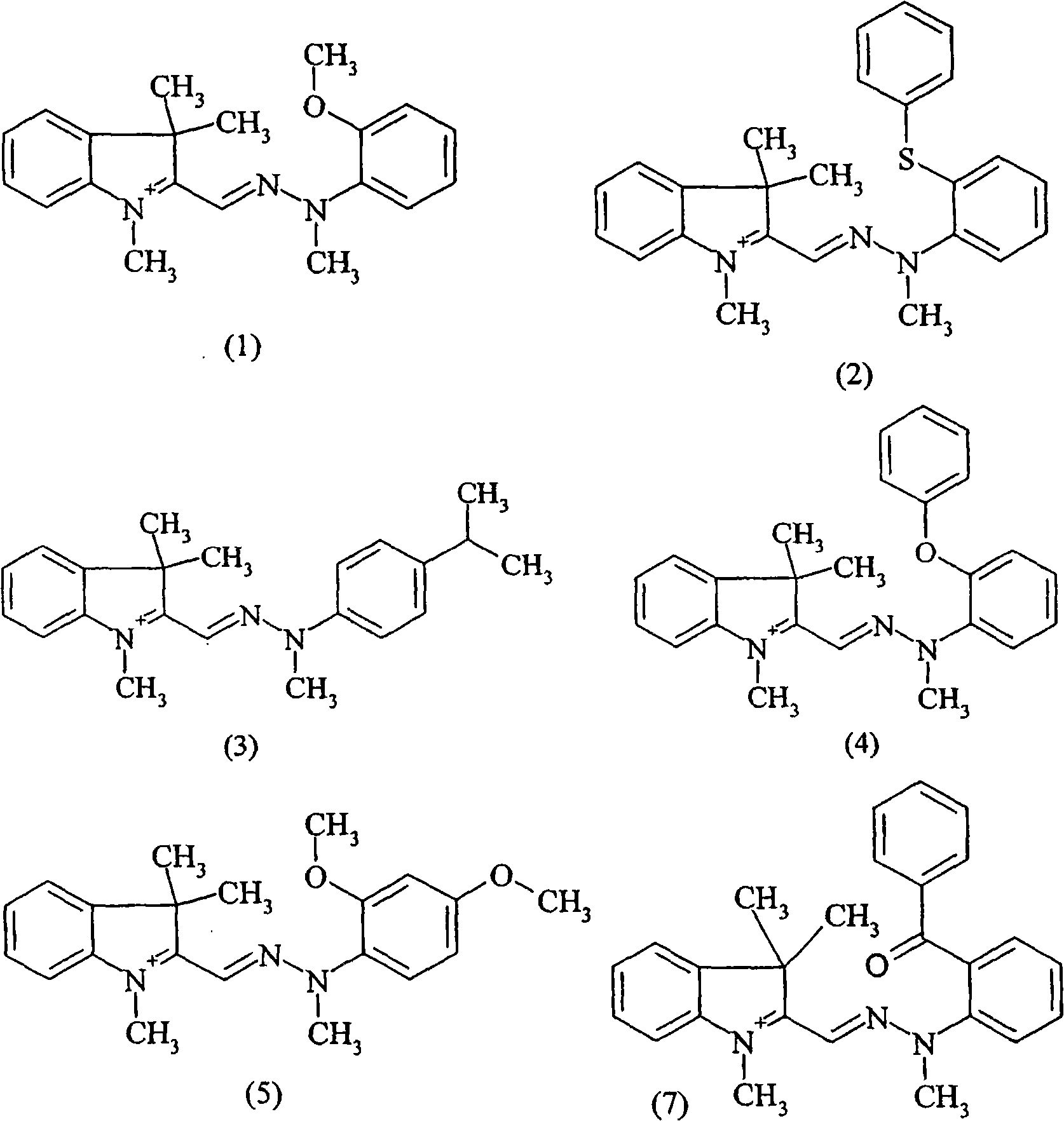
Use of indolinium diazamethine cations for optical data recording
A technology for optical data and applications, applied in the field of optical layers for optical data recording, can solve problems such as blocking dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Diazotization reaction and azo coupling reaction
[0230] 32.2 g of concentrated aqueous HCl were added dropwise to a solution consisting of 12.4 g of 2-methoxyaniline in 100 ml of water. The temperature was lowered to 0°C using an ice bath and 20.8 ml of aqueous sodium nitrite (33.3 wt %) was added dropwise while maintaining the temperature below 5°C. The resulting solution was stirred at 0°C for 1 hour and added dropwise at 10°C to a mixture of 17.6 g 1,3,3-trimethyl-2-methyl-indoline, 31.8 g Na 2 CO 3 , 100ml of methanol and 30ml of water in a mixture.
[0231] After complete addition, the resulting mixture was stirred at 10°C for 1 hour. Concentrated aqueous HCl was then added until pH=7. The resulting precipitate was filtered, washed with 1000 ml of water and air-dried to give 28.3 g of a yellow intermediate, namely the compound of formula (Vd_1).
[0232] Alkylation Method A
[0233] 28.3 g of the obtained compound of formula (Vd_1) are taken up in 200 ml of...
Embodiment 2 to 8
[0234] Embodiment 2 to 8: diazotization and coupling reaction
[0235] The corresponding aniline compounds are used to carry out the diazotization and coupling reactions according to Example 1 to obtain the formulas (2_1), Compounds of (3_I), (4_I), (1_Cl), (3_Cl), (5_Cl) and (7_I).
Embodiment 2 to 4 and 8
[0236] Examples 2 to 4 and 8: Alkylation Method A
[0237] The corresponding intermediates are used to carry out the alkylation according to Example 1. In cases where the desired final compound did not precipitate, the reaction mixture was evaporated to dryness and the compound was used without further purification, as was the case for example 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com