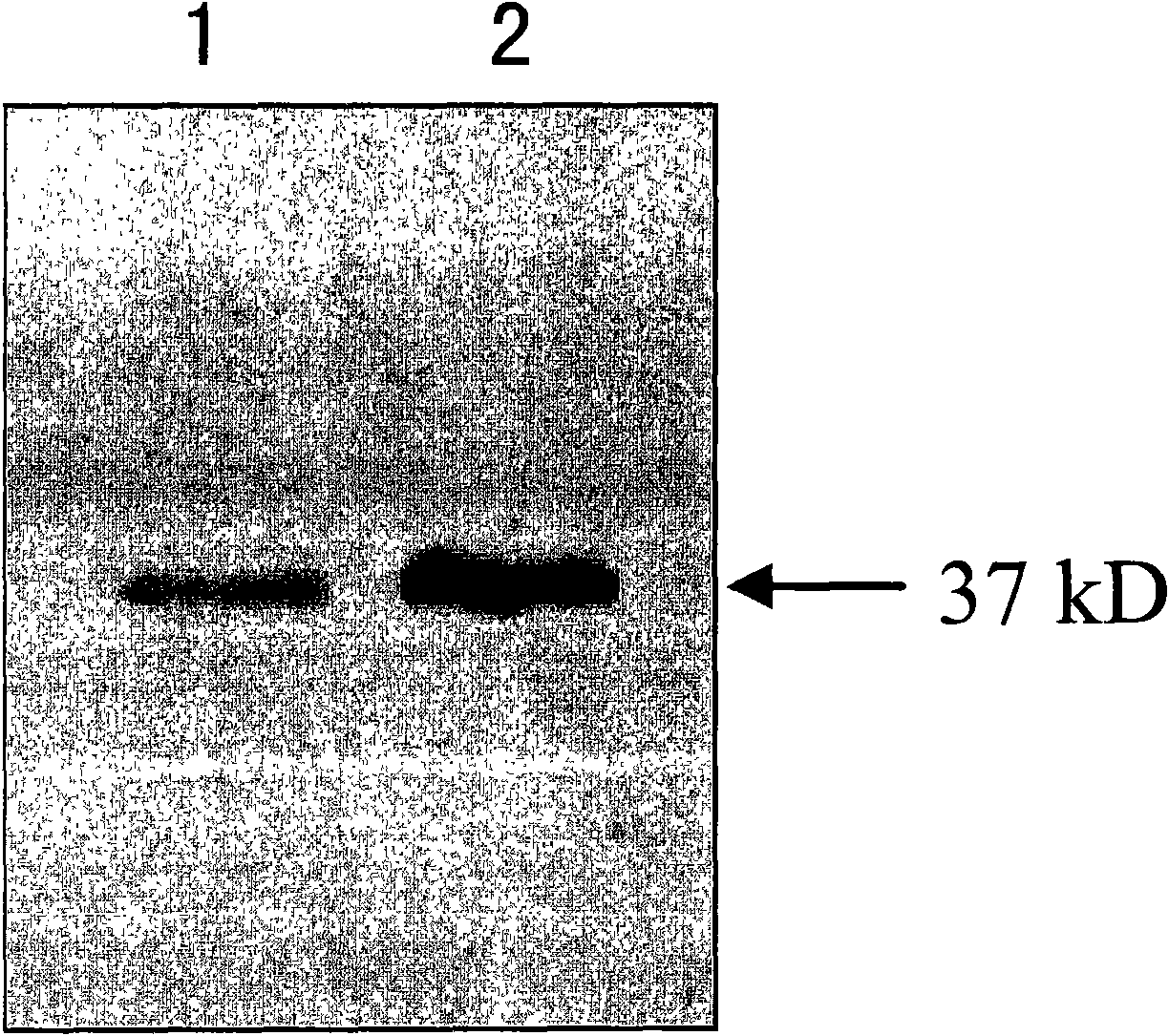Application of FHL 1 in preparing medicament for treating tumour
A technology of FHL1 and uses, which is applied in the field of use of FHL1 in the preparation of drugs for the treatment of tumors, and can solve the problems of unclear molecular mechanism and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
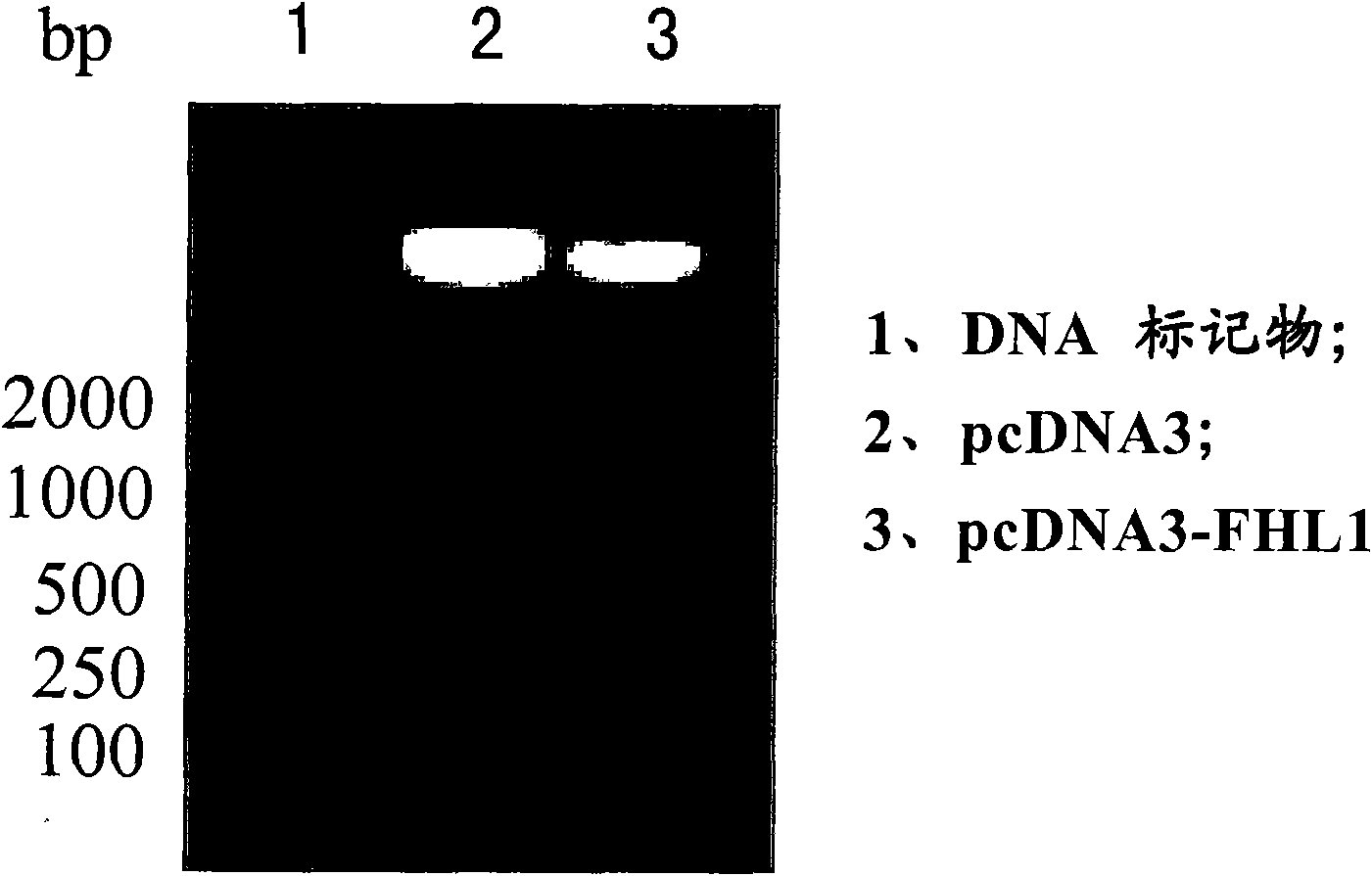
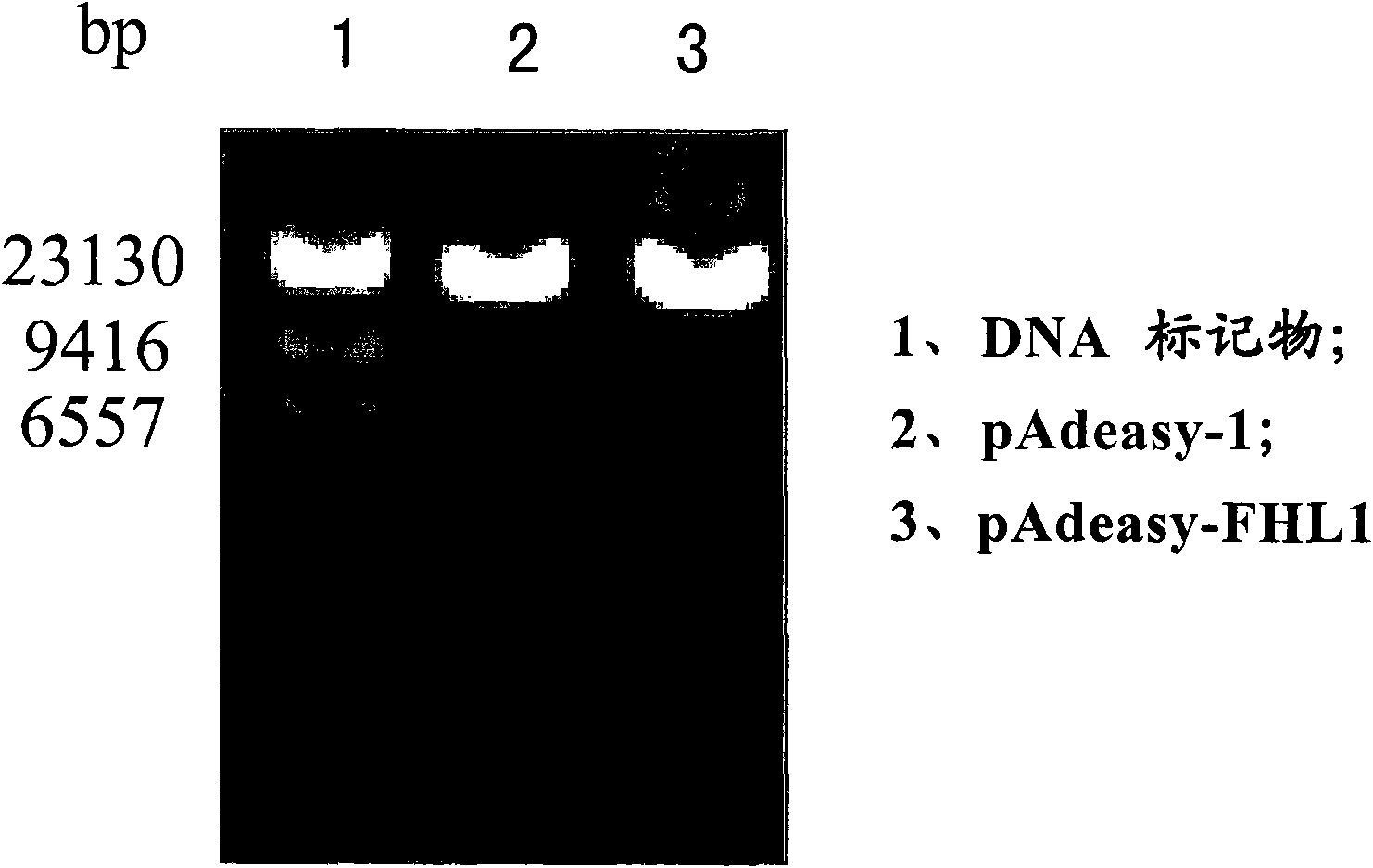
[0047] Embodiment 1, FHL1 mammalian cell expression vector and FHL1 recombinant adenovirus vector
[0048] (1) Method
[0049] 1. Construction of FHL1 mammalian cell expression vector
[0050] The complete coding region sequence of the FHL1 gene was amplified by PCR from a mammary gland cDNA library (purchased from Clontech, USA) according to conventional methods. : After 5 minutes at 94°C, 30 cycles of 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute, and finally 72°C for 7 minutes. Kpn I and Xba I enzymes (purchased from Biolabs, UK) were used to digest the PCR products and corresponding mammalian cell expression vectors (pcDNA3, purchased from Invitrogen, USA) at 37°C, and the PCR products and vectors were digested with T4 DNA ligase (purchased from Promega, USA) was ligated at 12-16°C to obtain a recombinant vector (which contains the CMV promoter / enhancer, the complete coding region sequence of the FHL1 gene, the BGH polyA termination signal, the ampicillin...
Embodiment 2
[0082] Example 2, FHL1 inhibits the growth of various tumor cells cultured in vitro
[0083] (1) Method
[0084]The effect of FHL1 on the growth of various tumor cells was determined by crystal violet assay, and the steps were as follows: Digest various tumor cells, including breast cancer cell lines (MCF7, ZR75-1, MDA-MB-468, MDA-MB231), liver cancer cell lines (HepG2, Hep3B, SMMC7721), lung cancer cell lines (A549, Calu3, H460), gastric cancer cell lines (BGC823, MKN-1). After counting the cells, dilute to an appropriate concentration, put 0.5ml of the cell solution into a 24-well plate, so that the number of cells in each well is about 10,000. Transfect these tumor cells with the FHL1 mammalian cell expression vector according to the method described in Example 1 or infect these tumor cells with 20-100 MOI of FHL1 recombinant adenovirus, after cultivating for 1-4 days, remove the medium, and add 0.5 ml to each well 1% glutaraldehyde (fixative solution), stand still fo...
Embodiment 3
[0087] Example 3, FHL1 inhibits anchorage-independent growth of various tumor cells
[0088] (1) Method
[0089] The effect of FHL1 on the anchorage-independent growth of tumor cells was determined by soft agar assay. First, the FHL1 mammalian cell expression vector was transfected into tumor cells according to the method described in Example 1 or the tumor cells were infected with FHL1 recombinant adenovirus at 20-100 MOI, including breast cancer cell lines (MCF7, ZR75-1, MDA-MB -468, MDA-MB231), liver cancer cell lines (HepG2, Hep3B, SMMC7721), lung cancer cell lines (A549, Calu3, H460), gastric cancer cell lines (BGC823, MKN-1), and then use 60mm 2 Cell culture dish, add 3ml DMEM medium containing 0.7% agar (purchased from U.S. Invitrogen Company) in the culture dish, add 3ml DMEM medium containing above-mentioned 20,000 tumor cells and 0.25% agar on it after solidification, each group The cells were set up in 3 parallel samples, and the number of colonies formed by c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com