Cysteic acid derivatives of anti-viral peptides
A cysteic acid and virus technology, applied in the direction of viral peptides, antiviral agents, viruses, etc., can solve the problems of poor solubility and short cytoplasmic half-life in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
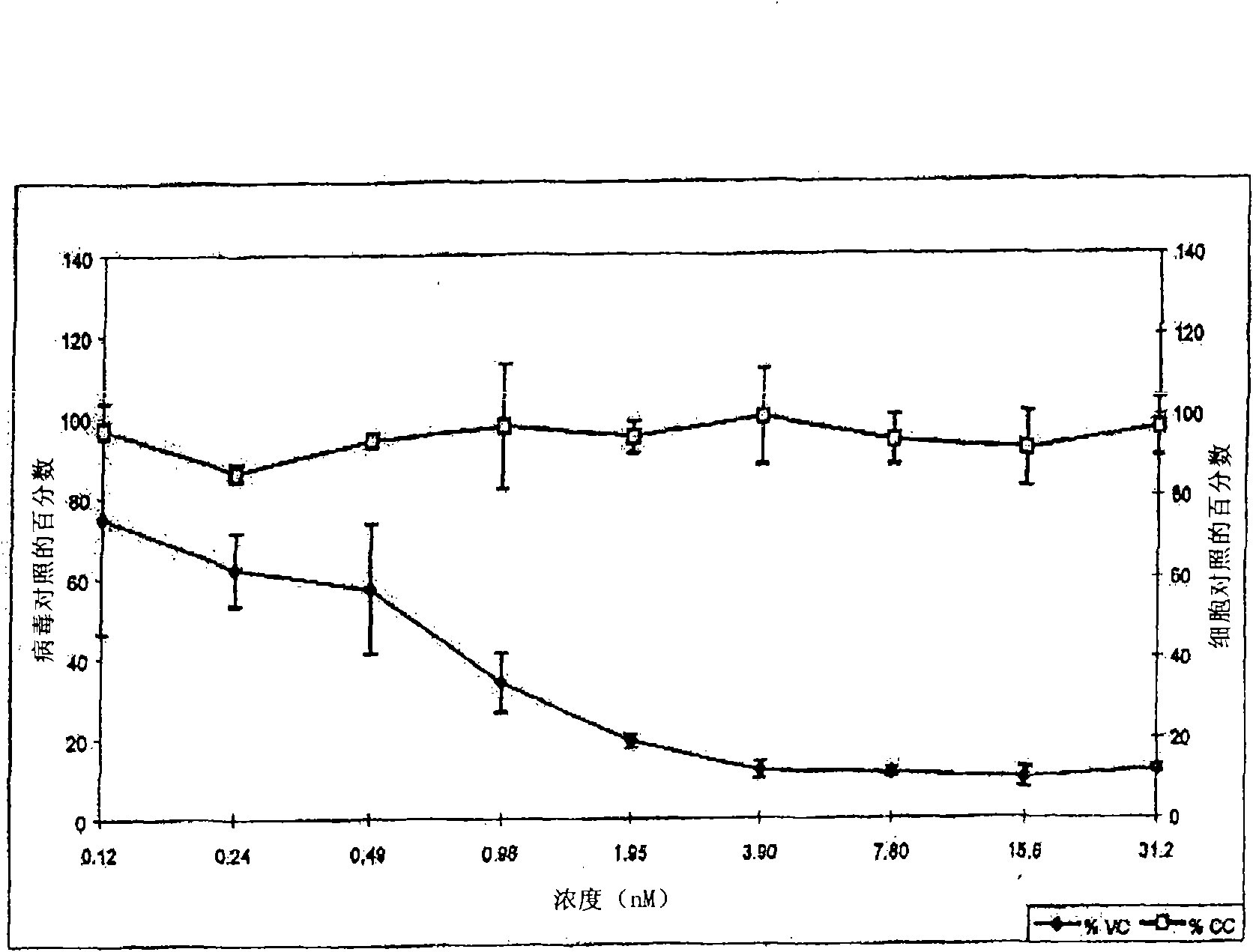
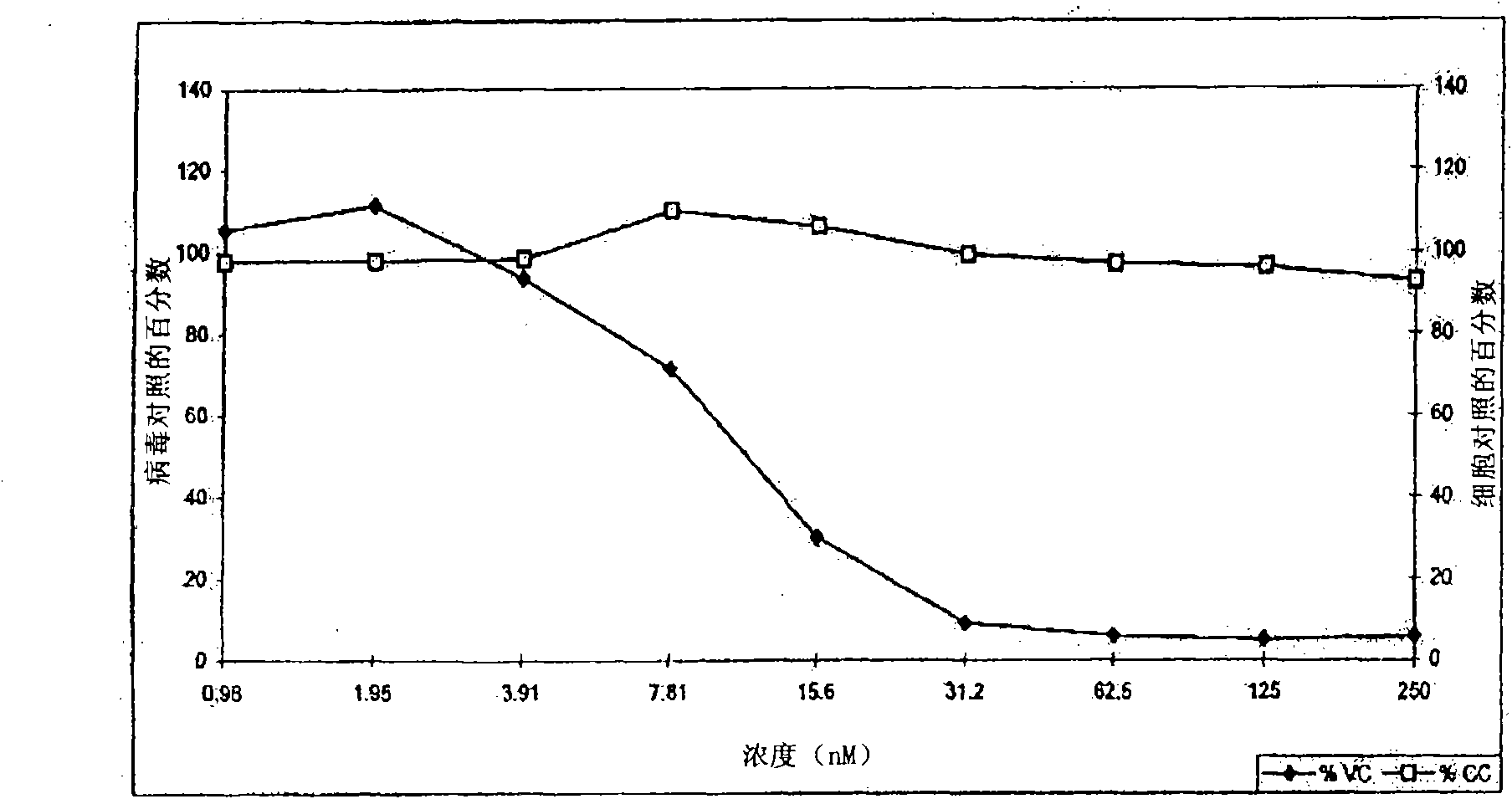
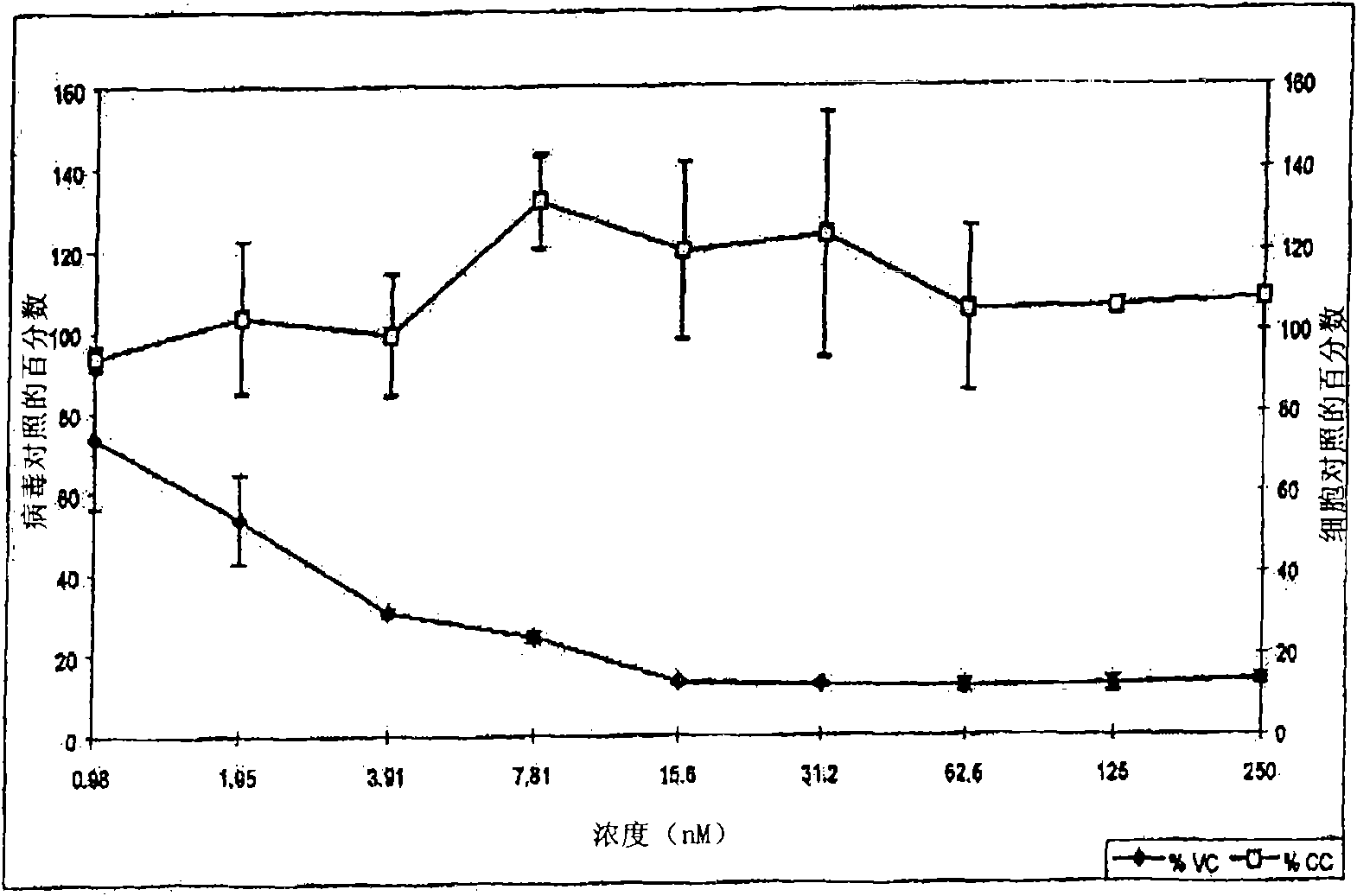
[0306] Example 1-5: Synthesis and purification of C34 cysteine sulfonic acid derivatives
[0307] Synthesis of cysteic acid derivatives of C34 was performed on a twelve-channel (Symphony) peptide synthesizer using an automated solid-phase method, with manual intervention during peptide generation. Synthesis was performed on Fmoc-protected Ramage amide linker resin using Fmoc-protected amino acids. The linkage uses O-benzotriazol-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) as an activator mixture in N , in N-dimethylformamide (DMF) solution. The Fmoc protecting group was removed using 20% piperidine / DMF. Boc-protected amino acids are used at the N-terminus to generate free N when the peptide is cleaved from the resin α - end. Sigmacote-treated glass reaction vessels were used during the synthesis.
Embodiment 2
[0308] Embodiment 2: the synthesis of CA-C34
[0309] CA-C34 has the following amino acid sequence:
[0310] Cysteine-Trp-Met-Glu-Trp-Asp-Arg-Glu-Ile-Asn-Asn-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln -Asn-Gln-Gln-Glu-Lys-Asn-Glu-Gln-Glu-Leu-Leu-CONH 2 (SEQ ID NO: 2). CA-C34 modified peptides were synthesized as follows:
[0311] Step 1: Solid-phase peptide synthesis of CA-C34 was performed on a 100 μm scale using manual and automated solid-phase synthesis, twelve-channel (Symphony) peptide synthesizer and Ramage resin. Add the following protected amino acids to the resin sequentially: Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(tBu)-OH , Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asn( Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Ile-OH, Fmoc-Leu -OH, Fmoc-Ser(tBu)-OH, Fmoc-His(Trt)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu...
Embodiment 3
[0313] Embodiment 3: CA-C34 (Arg 28 )Synthesis
[0314] CA-C34 (Arg 28 ) has the following amino acid sequence:
[0315] Cysteine-Trp-Met-Glu-Trp-Asp-Arg-Glu-Ile-Asn-Asn-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln - Asn-Gln-Gln-Glu-Arg-Asn-Glu-Gln-Glu-Leu-Leu-CONH2 (SEQ ID NO: 3).
[0316] Step 1: CA-C34 (Arg 28 ) for solid-phase peptide synthesis. Add the following protected amino acids to the resin sequentially: Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(tBu)-OH , Fmoc-Asn(Trt)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Asn( Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Glu(tBu)-OH, Fmoc-Ile-OH, Fmoc-Leu -OH, Fmoc-Ser(tBu)-OH, Fmoc-His(Trt)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH , Fmoc-Tyr(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Glu(tBu)-OH, Fmoc-Arg(Pbf)- OH, Fmoc-Asp(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Glu(tBu)-OH, Fmoc-M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com