Application of interleukin-1 receptor antagonist and medicinal composition thereof
一种药物、用途的技术,应用在白细胞介素-1受体拮抗剂的用途及其药物组合物领域,能够解决限制应用、安全性未被完全证实等问题,达到减少体重降低、改善不良反应、减少腹泻的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
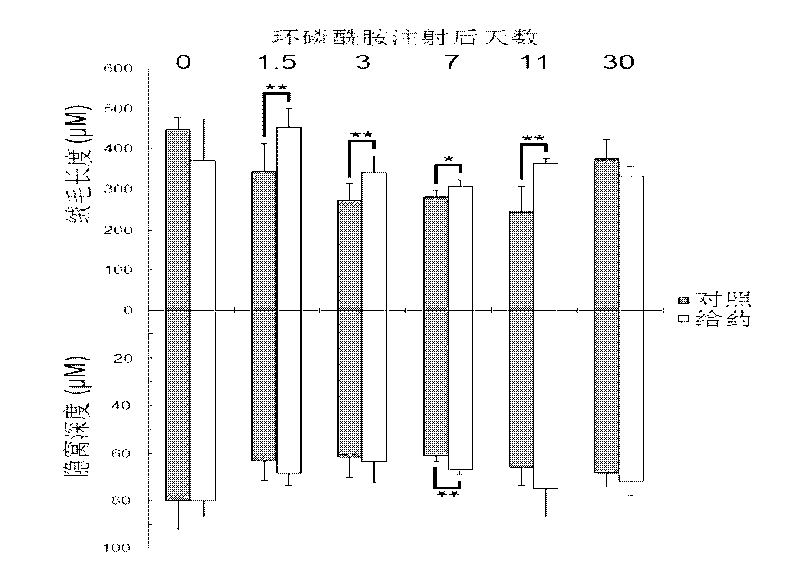
[0106] (Protective effect of IL-1Ra prophylactic drug on the small intestine of chemotherapy mice) Protective effect of IL-1Ra prophylactic drug on the small intestine in single-dose cyclophosphamide chemotherapy mice.
[0107] Balb / c mice, SPF grade for 8 weeks, weighing 23-28 grams, were randomly divided into two groups. The first group was injected with rhIL-1Ra (1 mg / kg b.w.I.P. every 24 days) for 3 consecutive days from Day-3 to Day-1. every hour), and the second group was injected with an equal volume of normal saline (I.P., every 24 hours). CTX was injected 12 hours after the last injection of protein or saline, once (300mg / kg b.w.I.P.). Observation indicators: HE staining of small intestine sections. The small intestine section at 15 cm below the stomach was taken from each mouse, and 4 sections were counted, 10 villi and 10 crypts were counted on each section, and the length was averaged.
[0108] result:
[0109] Small intestine HE staining statistics showed that ...
Embodiment 2
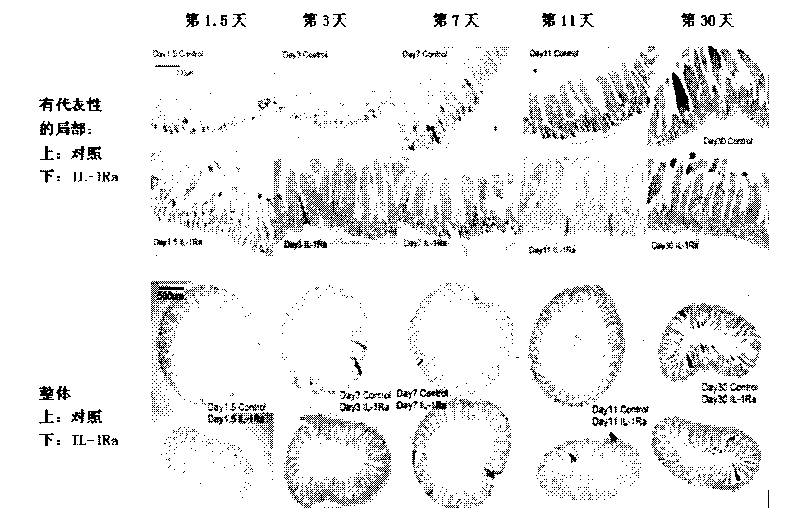
[0116] (Protective effect of IL-1Ra preventive drug on the small intestine of chemotherapy mice) Protective effect of IL-1Ra preventive drug on the small intestine of mice treated with cyclophosphamide chemotherapy for three consecutive days.
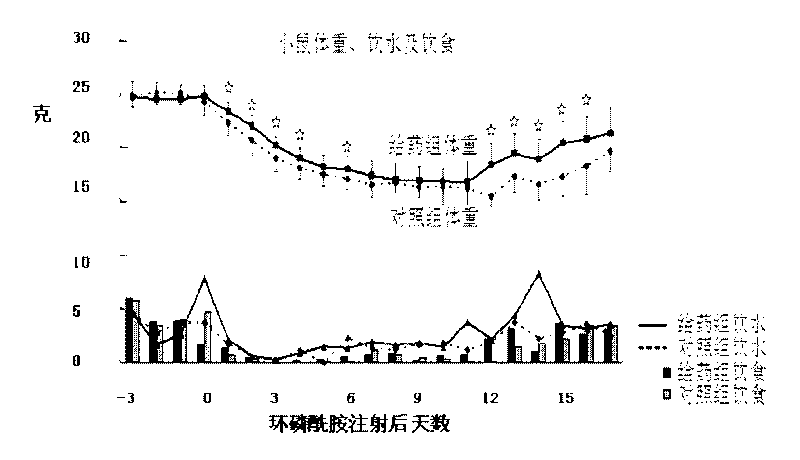
[0117] Balb / c mice, SPF grade for 8 weeks, body weight 23-28 grams, were randomly divided into two groups, the first group was injected with rhIL-1Ra (1 mg / kg b.w.I.P.) for 5 consecutive days from Day-5 to Day-1, every every 24 hours), and the second group was injected with an equal volume of normal saline (I.P., every 24 hours). CTX was injected 24 hours after the last injection of protein or saline, and three consecutive injections were made from Day 0 to Day 2 (200 mg / kg b.w.I.P., once every 24 hours). Observation indicators: body weight, food intake, water intake, diarrhea and death.
[0118] result:
[0119] Body weight: During the observation period from Day 0 and later, the mean body weight of the administration group was highe...
Embodiment 3
[0125] (Protective effect of IL-1Ra preventive medication on the small intestine of chemotherapy mice) Protective effect of IL-1Ra preventive medication on the small intestine of twice cyclophosphamide chemotherapy mice: Balb / c mice, SPF grade 8 weeks, body weight 23 ~30 grams, randomly divided into two groups, continuous intraperitoneal injection of 1 mg / kg RhIL-1Ra from Day-3 to Day-1, once a day for 3 consecutive days, the control group was injected with NS (Day-3 to Day-1); Day0 One-time intraperitoneal injection of 200mg / kg cyclophosphamide. One month later, IL-1Ra and CTX were injected for the second time, the method and dosage were the same as above, and the interval between the two CTX injections was 30 days. Observe the mouse body weight and survival rate after the second injection of CTX
[0126] result:
[0127] Body weight levels of mice after two cyclophosphamide chemotherapy (200mg / kg*2 times). During the experiment, the average body weight of the mice in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com