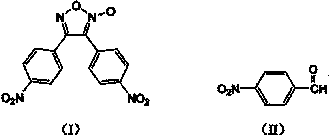
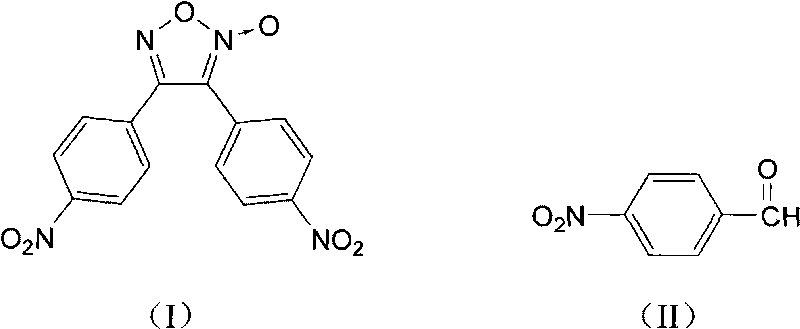
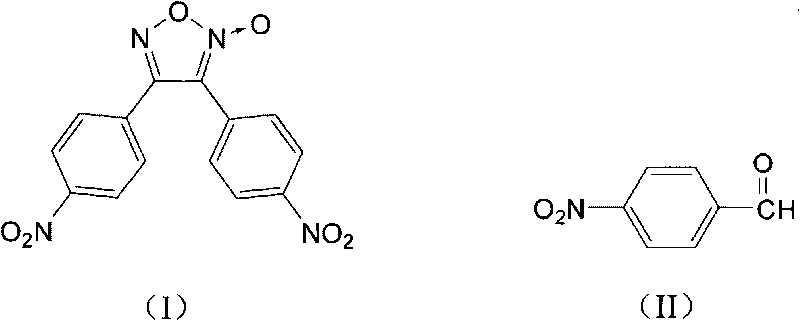
Synthesis method of 3,4-bi(4'-nitrophenyl) oxidized furoxan
A technology for oxidizing furoxan and nitrophenyl, which is applied in 3 fields and can solve the problems of many reaction steps and long reaction period
Inactive Publication Date: 2010-04-14
XIAN MODERN CHEM RES INST
View PDF0 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a synthesis method of 3,4-bi(4'-nitrophenyl) oxidized furoxan. The structure formula thereof is shown as (I), and the synthesis method adopts p-nitrobenzaldehyde as raw material; the structure formula thereof is shown as (II), and the synthesis method comprises the following steps: 1) adding the p-nitrobenzaldehyde and alcohol into a reaction flask, then adding hydroxylamine hydrochloride aqueous solution, using Na2CO3 aqueous solution to adjust pH of reaction liquid to 6 to 8, reacting for 1 to 2 hours at the temperature of 10 to 40 DEG C, obtaining p-nitrobenzaldoxime, with the molar ratio of the p-nitrobenzaldehyde and hydroxylamine hydrochloride of 1:1 to 1:1.5; and 2) adding the p-nitrobenzaldoxime, chlorosuccinimide and trichloromethane into the reaction flask, with the molar ratio of the p-nitrobenzaldoxime and chlorosuccinimide of 1:1 to 1:1.5, reacting for 1 to 3 hours at the temperature of 20 to 40 DEG C, using Na2CO3 solution to adjust pH of a system to 6 to 8 at the temperature of 0 to 5 DEG C, reacting for 1 to 4 hours and obtaining the objective product, namely the 3,4-bi(4'-nitrophenyl) oxidized furoxan. The synthesis method is mainly used for the 3,4-bi(4'-nitrophenyl) oxidized furoxan.
Description
technical field The invention relates to a synthesis method of 3,4-bis(4'-nitrophenyl)furazan. Background technique Furazan oxides are an important class of energetic compounds. Furazan oxide rings in the molecule as explosive groups can provide relatively higher energy density; furazan oxide derivatives can improve the oxygen balance of the system as energetic additives. Increase the detonation pressure; In addition, the furoxan ring can also endow its derivatives with ring tension and high standard enthalpy of formation. 3,-bis(4'-nitrophenyl)furoxan is an energetic material with good thermal stability and high energy density. The main synthesis method of 3,4-bis(4'-nitrophenyl)furoxan is dimerization of nitrile oxide. For example, "Synthesis and characterization of diarylfuroxans" (Defence Science Journal, 2006, 56, 4, 551-557) discloses a synthetic method of 3,4-bis(4'-nitrophenyl)furoxans, which uses p-Nitrobenzaldehyde is a raw material, and its synthetic route is ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D271/08
Inventor 吕剑薛云娜杨健民余奉侏李春迎谷玉杰杜咏梅刘波
Owner XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com