Method for synthesizing beta-damascenone
A synthesis method and technology of turkone are applied in the field of synthesis of β-turkone, which can solve the problems of flammability and explosion, unfavorable safety production, easy damping of metal hydrides, etc., and achieves easy operation, high reaction yield, and decomposition reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
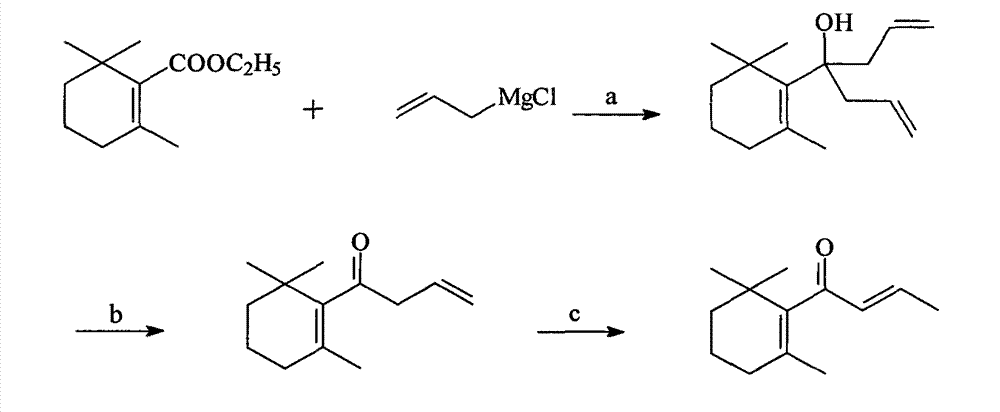
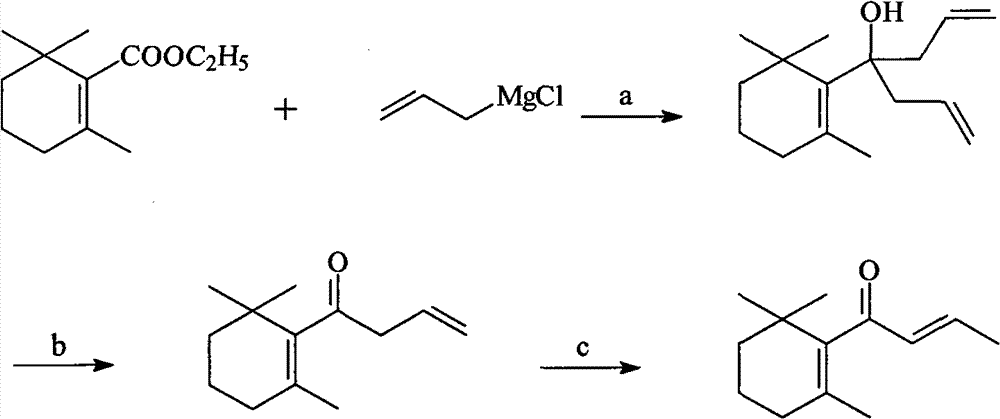
[0055] (1), Preparation of 2,6,6-trimethyl-1-(4-hydroxy-hept-1,6-dien-4-yl)-cyclohex-1-ene
[0056] In a 500-milliliter three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a spherical condenser, add 28.8 grams (1.2 mol) of magnesium, a grain of iodine and anhydrous ether (the addition amount is 100 milliliters), and the stirring speed is controlled to be 120r / min, start to drip 84.2g (1.1mol) of allyl chloride and anhydrous ether (addition amount is 100ml) mixed solution, control the rate of dripping to 5g / min, complete the dropwise addition, keep the reaction for 1h. Then, 100 g of ethyl cyclogeranate (0.5 mol) with a content of 98% was started dropwise, and the drop rate was controlled to be 6 g / min. After the dropwise addition was completed, stirring was continued for 2 h. The reaction solution was poured into 1000 mL of saturated aqueous ammonium chloride solution, the organic layer was separated, and washed once with 500 mL of water until neut...
Embodiment 2
[0062] (1), Preparation of 2,6,6-trimethyl-1-(4-hydroxy-hept-1,6-dien-4-yl)-cyclohex-1-ene
[0063] In a 500-milliliter three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a spherical condenser, add 28.8 grams (1.2 mol) of magnesium, a grain of iodine and isopropyl ether (the addition amount is 100 milliliters), and the stirring speed is controlled to be 120r / min, start to drip 84.2g (1.1mol) of allyl chloride and isopropyl ether (addition amount is 100ml) mixed solution, control the rate of dripping to 5g / min, complete the dropwise addition, keep the reaction for 1h. Then, 100 g of ethyl cyclogeranate (0.5 mol) with a content of 98% was started dropwise, and the dropping rate was controlled to be 6 g / min. After the dropwise addition was completed, stirring was continued for 3 h. The reaction solution was poured into 1000 mL of saturated aqueous ammonium chloride solution, the organic layer was separated, and washed once with 500 mL of water until ...
Embodiment 3
[0069] (1), Preparation of 2,6,6-trimethyl-1-(4-hydroxy-hept-1,6-dien-4-yl)-cyclohex-1-ene
[0070] In a 500-milliliter three-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a spherical condenser, add 28.8 grams (1.2 mol) of magnesium, a grain of iodine and anhydrous ether (the addition amount is 100 milliliters), and the stirring speed is controlled to be 120r / min, start to drip 84.2g (1.1mol) of allyl chloride and anhydrous ether (addition amount is 100ml) mixed solution, control the rate of dripping to 5g / min, complete the dropwise addition, keep the reaction for 1h. Then, 100 g of ethyl cyclogeranate (0.5 mol) with a content of 98% was started dropwise, and the dropping rate was controlled to be 6 g / min. After the dropwise addition was completed, stirring was continued for 3 h. The reaction solution was poured into 1000 mL of saturated aqueous ammonium chloride solution, the organic layer was separated, and washed once with 500 mL of water until ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com