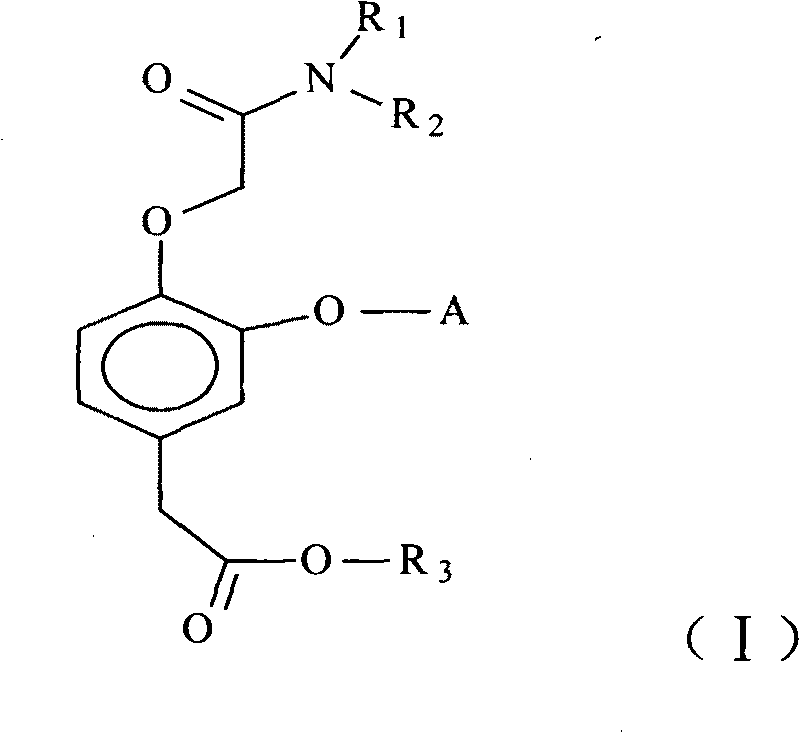
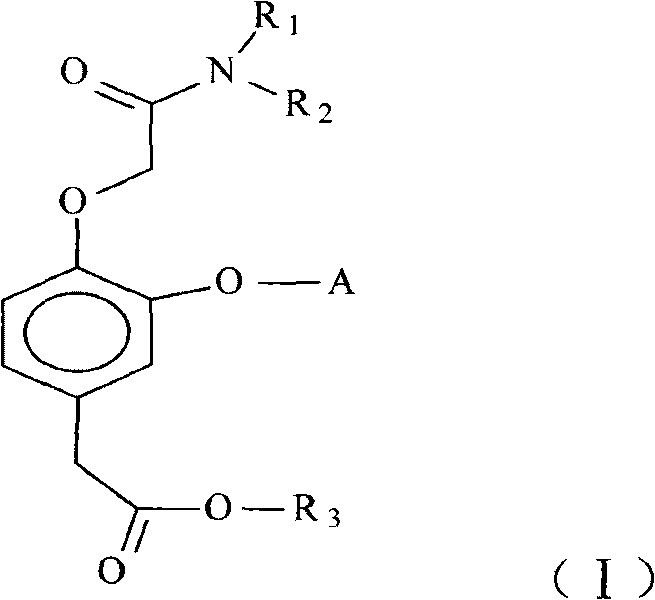
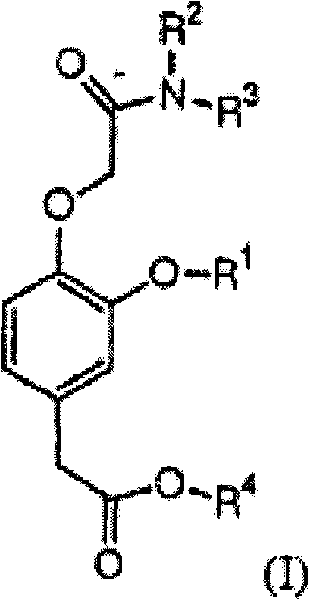
Short-acting hypnotizing and calming compound used for anesthetization
A compound and alkyl technology, applied in the directions of active ingredients of heterocyclic compounds, anesthetics, organic chemistry, etc., can solve the problems of poor efficacy and insufficient anesthetic effect, and achieve low toxicity and side effects, predictable recovery time, and strong anesthetic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1.1, the synthetic technique of (3-trifluoromethoxy-4-hydroxyl) n-propyl phenylacetate (compound a):
[0078]
[0079] Compound a can be prepared by the following reaction scheme:
[0080]
[0081] 1.1.1 Compound a 1 The synthesis process:
[0082] Add 35.6g of 2-trifluoromethoxyphenol, 37ml of glyoxylic acid (40%), and 60ml of purified water into a 500ml three-necked flask equipped with mechanical stirring and a thermometer, cool in an ice bath to below 10°C, and slowly add 10 % sodium hydroxide solution 160ml, and maintain this temperature, add sodium hydroxide solution, temperature rises slowly to room temperature, maintain room temperature reaction 20 hours. The solution was extracted with 2×50ml ethyl acetate, then adjusted to pH 3 with concentrated hydrochloric acid, saturated with NaCl, extracted with 3×100ml ethyl acetate, the ethyl acetate layer was washed with 3×100ml saturated brine, anhydrous MgSO 4 Dry overnight, evaporate the solvent under reduced...
Embodiment 2
[0103] 2.1, Synthetic process of [3-(1.1.2.2-tetrafluoroethoxy)-4-hydroxy]n-propyl phenylacetate (compound b):
[0104]
[0105] 2.1.1 Compound b 1 The synthesis process:
[0106]
[0107] Using 2-(1.1.2.2-tetrafluoroethoxy)phenol instead of 2-trifluoromethoxyphenol, it was prepared using a similar process as described in Example 1.1.1. With 2-(1.1.2.2-tetrafluoroethoxy)phenol 42g, compound b was obtained 1 39.5g.
[0108] Elemental analysis result: C 10 h 8 f 4 o 5 C 42.25 H 2.82
[0109] Mass Spectrometry Results: M / Z[M+H + ]284
[0110] 2.1.2 Compound b 2 The synthesis process:
[0111]
[0112] It was prepared using a similar process as described in Example 1.1.2. Compound b from the previous step 1 28.5g, to obtain compound b 2 30.5g.
[0113] Elemental analysis result: C 14 h 12 f 4 o 7 C 45.65 H 3.27
[0114] Mass Spectrometry Results: M / Z[M+H + ]368
[0115] 2.1.3 Compound b 3 The synthesis process:
[0116]
[0117] It was prepa...
Embodiment 3
[0130] 3.1, the synthetic technique of [3-(2.2.2-trifluoroethoxy)-4-hydroxy]n-propyl phenylacetate (compound c):
[0131]
[0132] 3.1.1 Compound c 1 The synthesis process:
[0133]
[0134] It was prepared by a similar process described in Example 1.1.1 by substituting 2-(2.2.2-trifluoroethoxy)phenol for 2-trifluoromethoxyphenol. With 2-(2.2.2-trifluoroethoxy)phenol 38.5g, compound c was obtained 1 34g.
[0135] Elemental analysis result: C 10 h 9 f 3 o 5 C 45.10 H 3.40
[0136] Mass Spectrometry Results: M / Z[M+H + ]266
[0137] 3.1.2 Compound c 2 The synthesis process:
[0138]
[0139] It was prepared using a similar process as described in Example 1.1.2. Compound c from the previous step 1 26.6g, to obtain compound c 2 25.5g.
[0140] Elemental analysis result: C 14 h 13 f 3 o 7 C 47.99 H 3.73
[0141] Mass Spectrometry Results: M / Z[M+H + ]350
[0142] 3.1.3 Compound c 3 The synthesis process:
[0143]
[0144] It was prepared using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com