Nitrated cellulose grafted by spirooxazine group and preparation method thereof
A technology of nitrocellulose and spirooxazine, applied in the direction of chemical instruments and methods, color-changing fluorescent materials, etc., can solve problems such as phase separation, and achieve the effect of excellent performance and good photochromic performance
Inactive Publication Date: 2010-05-05
SHAANXI TECHN INST OF DEFENSE IND
View PDF0 Cites 13 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
On the other hand, nitrocellulose has broad application value in the field of coatings. Although the physical mixing of spirooxazine compounds in nitrocellulose can also obtain coatings with photochromic properties, because the two are heterogeneous at the molecular level phase system, there will be shortcomings such as phase separation during long-term use; Zhu Yuqin, Tang Liegui and others from the Guangzhou Institute of Chemistry, Chinese Academy of Sciences have reported grafted formazan in the literature: Applied Chemistry, 1993, 10 (6), 61-65 The film properties of the nitrocellulose based on methyl acrylate have reached the coating requirements without adding any plasticizers and modifiers.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
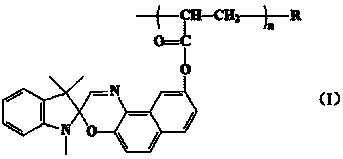
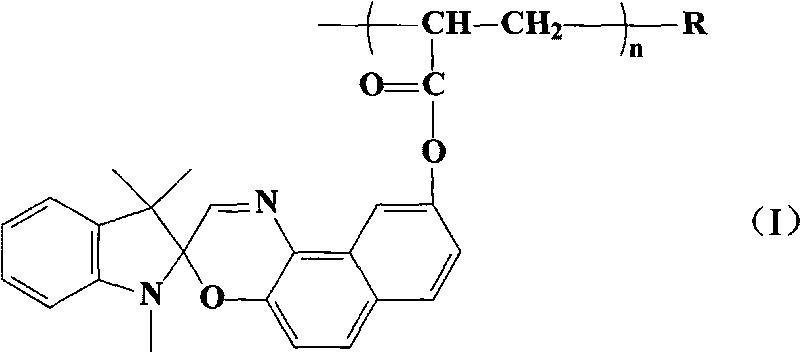
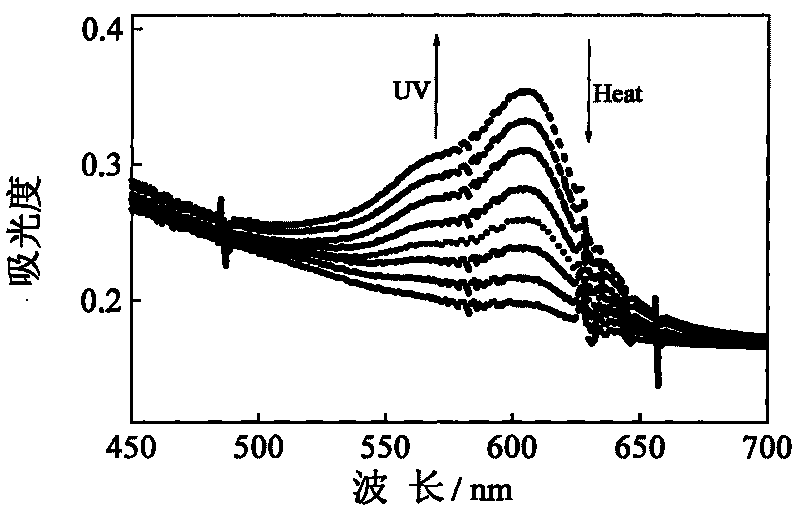
The invention discloses a nitrated cellulose grafted by spirooxazine group, which is shown by structural formula (I), wherein R is nitrated cellulose, and n is equal to 1-100; and the invention also discloses a preparation method of the nitrated cellulose, which comprises: dissolving the nitrated cellulose into methylisobutylketone, and obtaining mixture A; adding acrylic ester dye monomer containing the spirooxazine group into the mixture A, filling nitrogen for protection, evenly stirring, heating up to 70 DEG C, adding benzoyl peroxide for initiating polymerization reaction, reacting for 3.0h, cooling in ice water bath, and obtaining mixture B; using petroleum ether to lead impure graft to be precipitated, and using benzene to extract the graft for 48.0h in a Soxhlet extractor; and finally, removing dye homopolymer, carrying out vacuum drying at 60 DEG C, and obtaining high polymer of the nitrated cellulose. The high polymer has good photochromic performance, and can be used for preparing a photochromic film and coating. On the molecular level, the nitrated cellulose is a homogeneous system, and the spirooxazine can not be separated from the nitrated cellulose after a long-term use.
Description
technical field The invention belongs to the field of spirooxazine photochromic polymer materials, and in particular relates to nitrocellulose grafted with spirooxazine groups and a preparation method thereof. Background technique Photochromism refers to a compound that is irradiated by a certain wavelength of light and undergoes a specific chemical reaction to produce a product. Its absorption spectrum changes significantly, and it returns to its original form under the action of another wavelength of light or heat. Spirooxazine is a class of photochromic compounds with excellent photochemical properties, but the thermal stability of the ring-opening body of small molecule spirooxazine compounds is poor during the photochromic process, and small molecule spirooxazine compounds are limited in the manufacture of devices. The preparation of spirooxazine compounds as polymers with photochromic properties has obvious advantages in practical applications. At the same time, schol...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08F251/02C08B15/06C08F120/36C09K9/02
Inventor 孙宾宾刘耀鹏杨秀霞王芳宁杨博
Owner SHAANXI TECHN INST OF DEFENSE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com