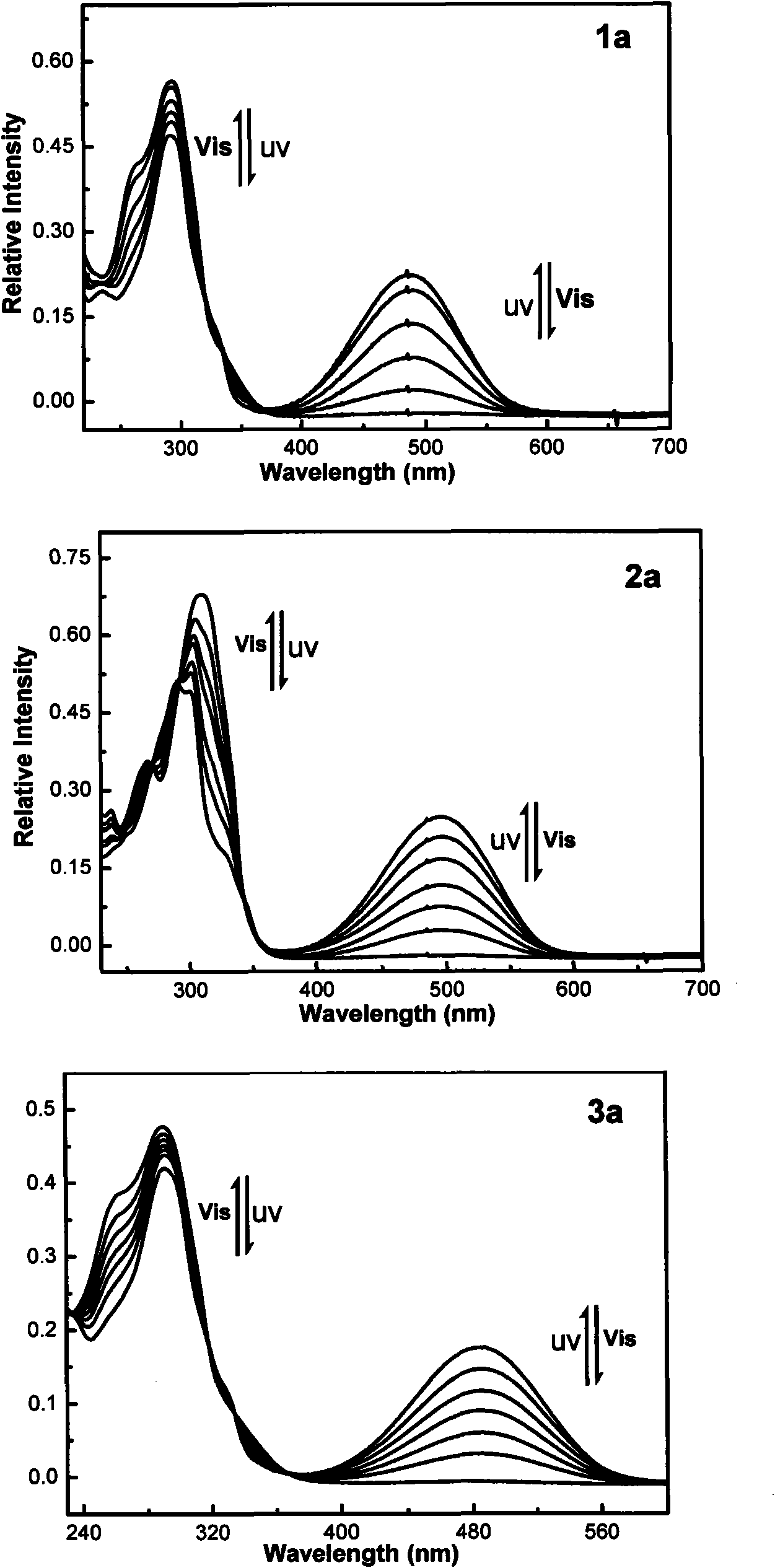
Photochromic thiophene-thiazole heterocycle hybrid asymmetric perfluorocyclopentene compound, preparation method and application
A perfluorocyclopentene, photochromic technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problem that the use has not yet been reported, and achieve good photochromic performance and significant fatigue resistance. , the effect of good chemical and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] [Example 1]: [Compound 1a]:
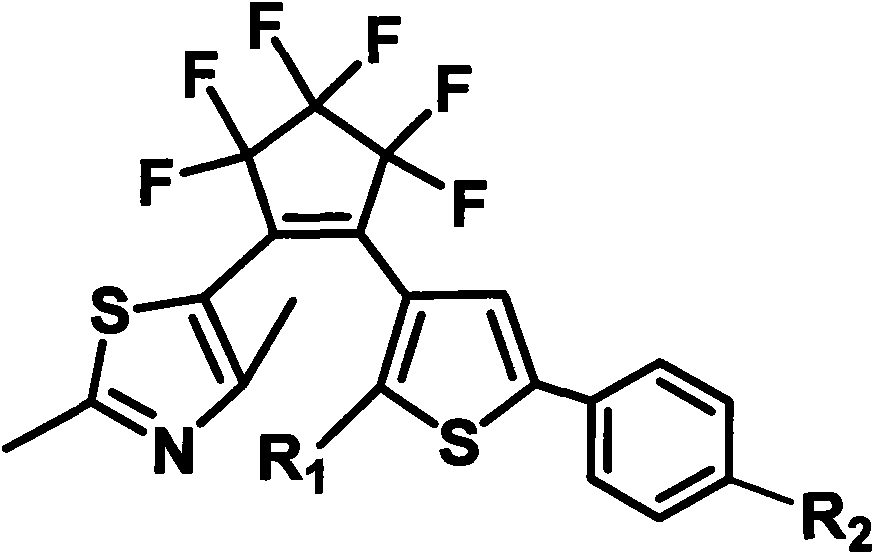
[0025] In the molecular structure formula, when R 1 is methyl, R 2 When it is a hydrogen atom, it constitutes the photochromic compound 1a, and its name is: [1-(2,4-dimethylthiazol-5-yl), 2-[2-methyl-5-phenylthiophene-3 -base)] perfluorocyclopentene (1a), the structural formula is as follows:
[0026]
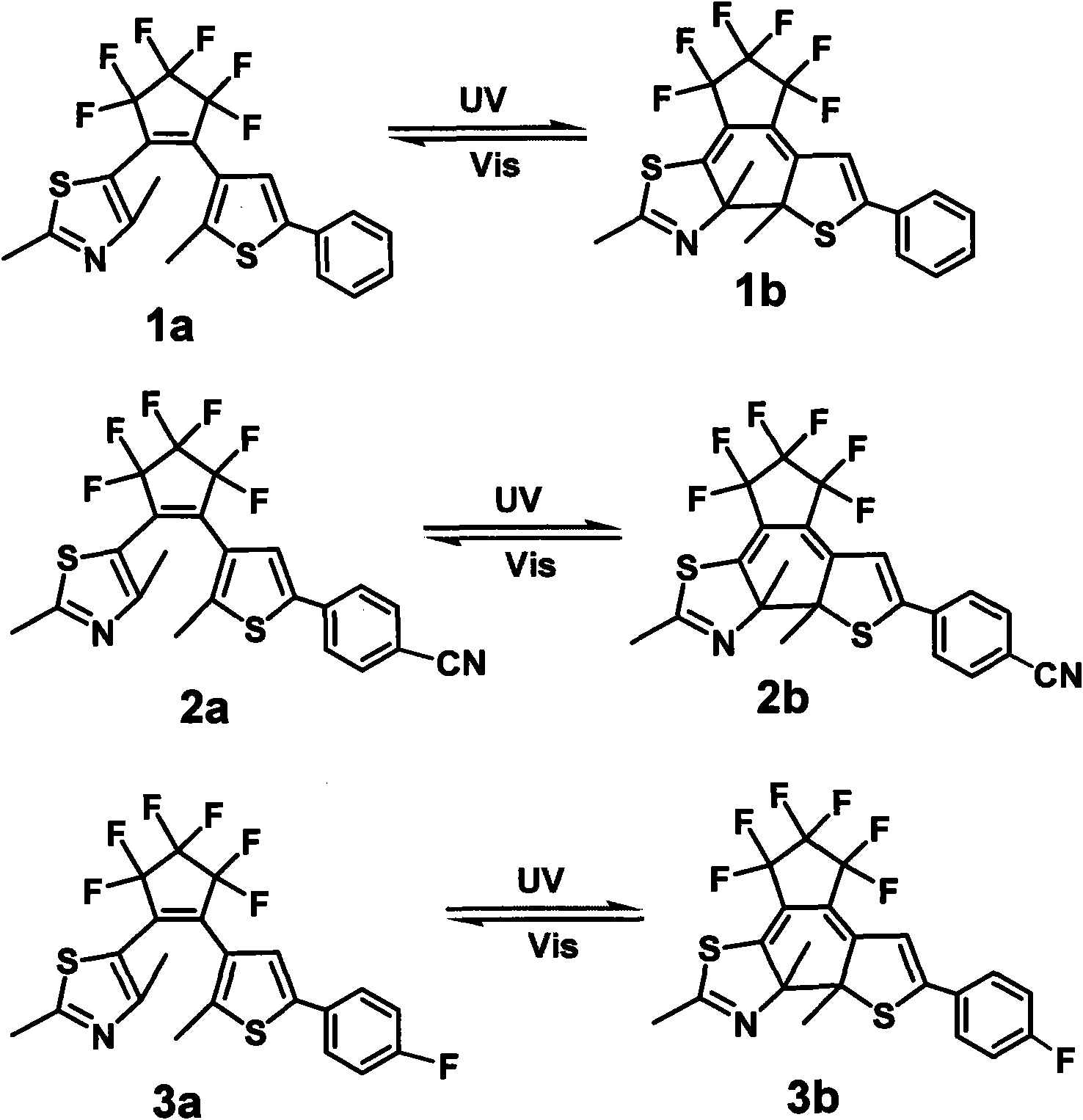
[0027] The synthetic scheme Scheme 1 of this novel diaryl perfluorocyclopentene photochromic compound is shown in Scheme 1: Scheme 1:
[0028]
[0029] Concrete synthetic steps are as follows:
[0030] 1.3,5-Dibromo-2-methylthiophene (5)
[0031] Under ice-bath conditions, dissolve 2-methylthiophene (4) (25.617g, 261.4mmol) in acetic acid, add dropwise acetic acid containing liquid bromine under stirring, continue the ice-bath reaction for 8h, add water, water Na 2 CO 3 After neutralization, extract with ether, combine the organic phases, and wash with saturated Na 2 CO 3 and aqueous solution successively, anhydrous MgSO 4 Dr...
Embodiment 2
[0048] [Example 2]: [Compound 2a]:
[0049] In the molecular structure formula, when R 1 is methyl, R 2 When it is a para-nitrile group, it constitutes a photochromic compound 2a, and its name is: [1-(2,4-dimethylthiazol-5-yl), 2-[2-methyl-5-(4- Nitrile phenyl) thiophen-3-yl)] perfluorocyclopentene (2a), the structural formula is as follows:
[0050]
[0051] The synthetic scheme Scheme 2 of this novel diaryl perfluorocyclopentene photochromic compound is shown in Scheme 2: Scheme 2:
[0052]
[0053] Concrete synthetic steps are as follows:
[0054] 1.3-Bromo-2-methyl-5-(4-cyanophenyl)thiophene (11)
[0055] Under nitrogen protection, 4-cyanobromobenzene (2.5g, 13.74mmol) and Pd(PPh 3 ) 4 (0.5g) was dissolved in 80mlTHF, after stirring for 20min, 6 (3.10g, 14mmol) was added and the concentration was 2.0mol / L Na 2 CO 3 Solution 50ml, heated to reflux for 16h, stop the reaction, cooled to room temperature. The liquids were separated, the aqueous phase was extract...
Embodiment 3
[0060] [Example 3]: [Compound 3a]:
[0061] In the molecular structure formula, when R 1 is methyl, R 2 When it is para-fluorine, it constitutes photochromic compound 3a, and its name is: [1-(2,4-dimethylthiazol-5-yl), 2-[2-methyl-5-(4-fluoro Phenyl) thiophen-3-yl)] perfluorocyclopentene (3a), the structural formula is as follows:
[0062]
[0063] The synthetic scheme Scheme 3 of this novel diaryl perfluorocyclopentene photochromic compound is shown in Scheme 3: Scheme 3:
[0064]
[0065] Concrete synthetic steps are as follows:
[0066] 1. 3-Bromo-2-methyl-5-(4-methoxyphenyl)thiophene (12)
[0067] Under nitrogen protection, 4-methoxybromobenzene (1.24g, 7.07mmol) and Pd(PPh 3 ) 4 (0.4g) was dissolved in 70mlTHF, after stirring for 20min, 6 (1.59g, 7.20mmol) was added and the concentration was 2.0mol / LNa 2 CO 3Solution 30ml, heated to reflux for 16h, stop the reaction, cooled to room temperature. The liquids were separated, the aqueous phase was extracted wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com