Application of cumarins compound in preparing medicine for inhibiting tumor growth
A coumarin, growth inhibitory technology, applied in the field of medicine, can solve the problems of no reports of biological activity, no tumor growth inhibitory drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Molecular docking and pre-assessment of activity
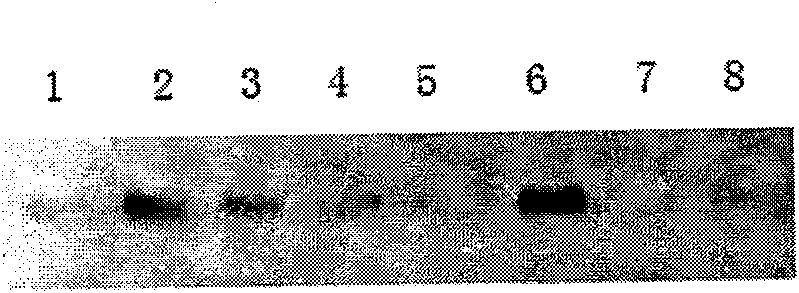
[0045] The present invention determines the key regions and key amino acid residues of the interaction between the oncoprotein gankyrin and the tumor suppressor protein Rb by studying the action mode of the two. The GST-pulldown experiment was used to analyze the changes in the binding ability of the constructed Rb key amino acid residue mutants to gankyrin, such as figure 1 shown, all 4 mutants can affect P28 to varying degrees GANK For binding with Rb, the effect of T5 (Y709F, K713A) and T10 (Y709A, K713A) is better. At the same time, the negative control GST-null and Rb-deficient cell line Saos2 lysate has no band, and PLC cell lysate has a band. Both T5 and T10 are mutated at sites 709 and 713, suggesting that these two sites play a key role in their binding. Small molecule compounds designed mainly for these two sites will improve the hits of positive compound screening. Rate.
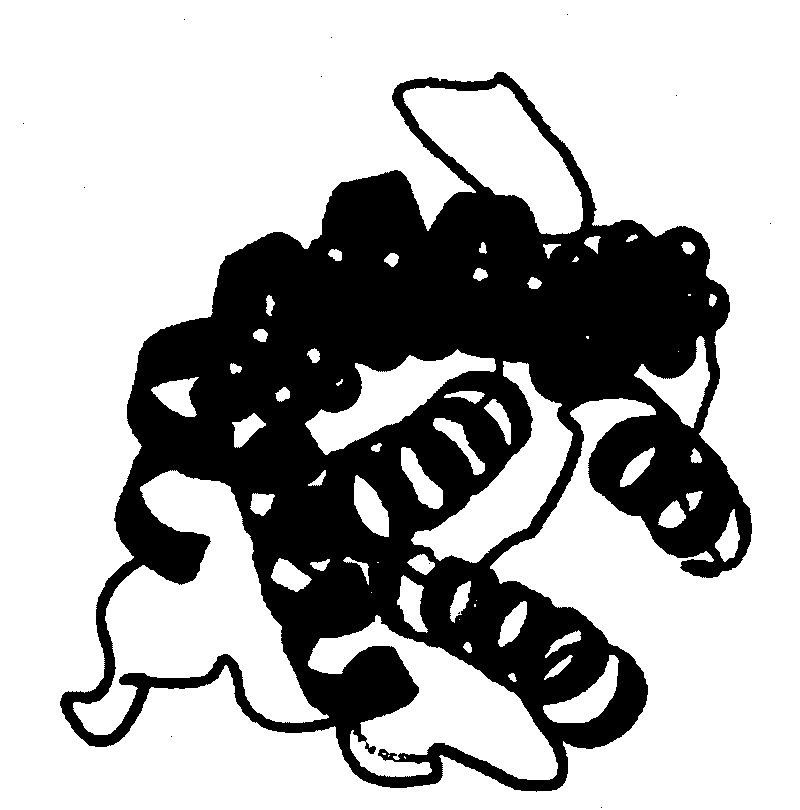
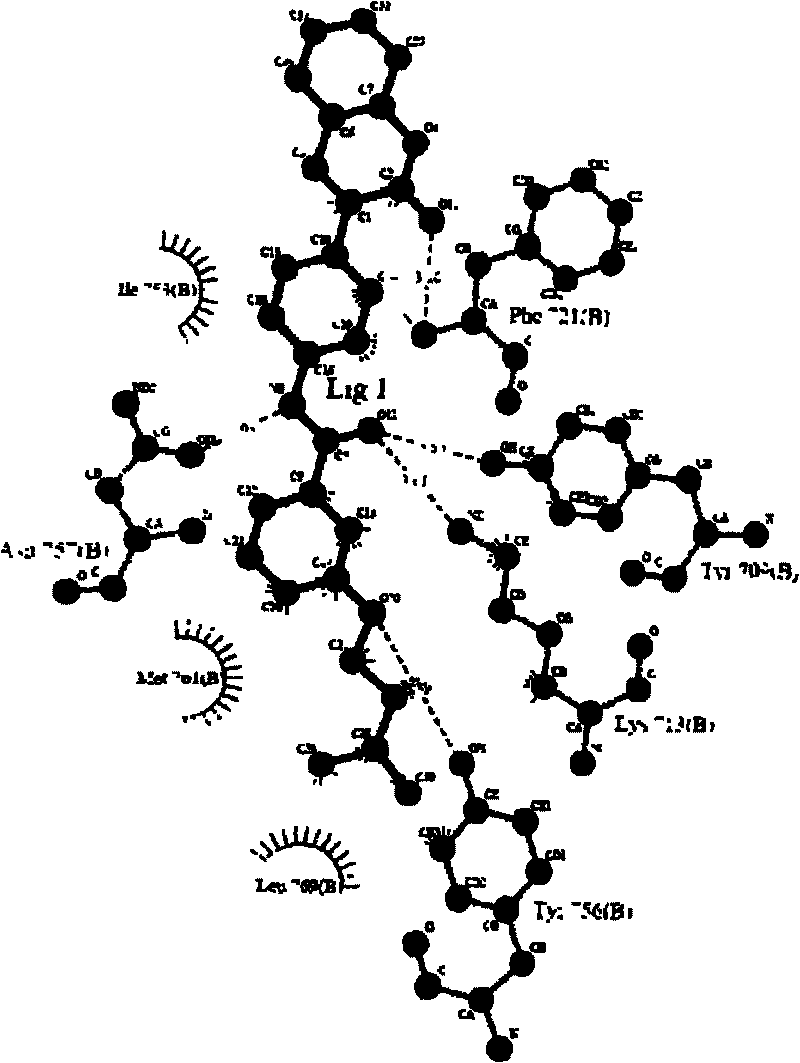
[0046] Then, the crys...
Embodiment 2
[0047] Example 2: Preparation of 3-[4-(3-isoamyloxy)benzamido]coumarin and its derivatives
[0048] Preparation of 13-[4-(3-isoamyloxy)benzamido]coumarin
[0049] (1) Preparation of 3-(p-nitrophenyl)coumarin
[0050]
[0051] Add 1g of 2-hydroxybenzaldehyde and 1.72g of p-nitrophenylacetic acid into a dry 50ml conical flask, add 18ml of acetic anhydride while stirring, and slowly add 460mg of NaH to the mixture, and react at room temperature for 3 hours , there is precipitation, suction filtration. Obtained product 1.8g, yield 82.5%
[0052] (2) Preparation of 3-(p-aminophenyl)coumarin
[0053]
[0054] Add Pd / C into a 250ml three-necked bottle, slowly drop the aqueous solution of sodium borohydride into the bottle under nitrogen protection, and slowly drop the methanol solution of 3-p-nitrophenylcoumarin into the bottle. into the reaction solution. Stir at room temperature for 2h. Filtration, the filtrate was evaporated to dryness, and separated to obtain 1.35 g o...
Embodiment 23
[0061] Example 23 Preparation of 3-[4-(3-benzyloxy)benzamido]coumarin
[0062] The isopentyl bromide in Example 1(3) was replaced with benzyl bromide, and the other operations were carried out as in Example 1 to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com