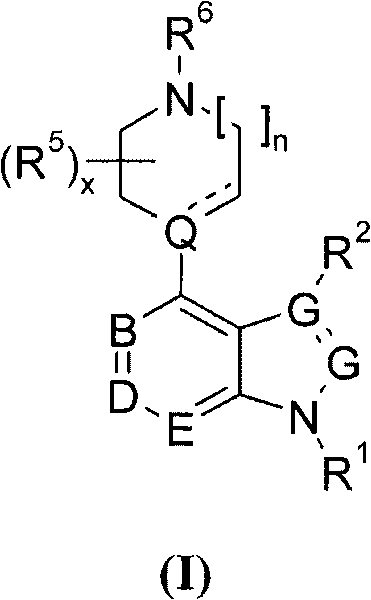
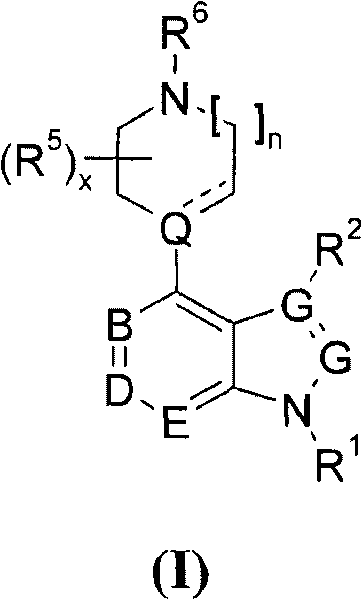
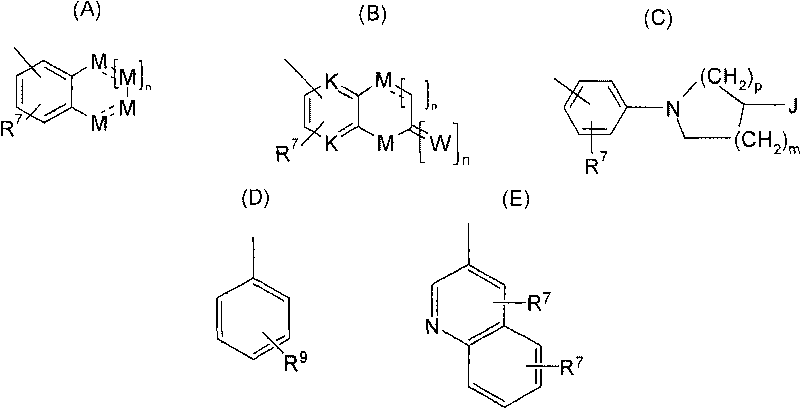
4' substituted compounds having 5-ht6 receptor affinity
A compound and solvate technology, applied in the field of compounds with selective 5-HT6 affinity, can solve the problem of selective agonists and antagonists hindering receptor function research in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] 4-methyl-7-[(4-piperazin-1-yl-1H-indol-1-yl)sulfonyl]-3,4-dihydro-2H-1,4-benzoxazine, (1) Preparation
[0264]
[0265] step 1. The starting compound 4-(1H-indol-4-yl)-piperazine-1-carboxylic acid tert-butyl ester [(A) 2.00×10 2mg, 0.000664 mol] was mixed together with tetrahydrofuran (1.0 mL, 0.01 mol) and N,N-dimethylformamide (1 mL, 0.015 mol) in a vial. The mixture was stirred at 0°C for 10 minutes. Under nitrogen atmosphere, sodium bis(trimethylsilyl)amide (1.0 mL of a 1M solution) in tetrahydrofuran was added via syringe and the resulting mixture was stirred for 10 minutes, to which was added 4-methyl-3 in one portion, 4-Dihydro-2H-1,4-benzoxazine-7-sulfonyl chloride (246 mg, 0.000995 mol). The reaction mixture was stirred for 3 hours, after which time LC-MS (8080_8 min) indicated that the reaction was complete. Solvent was removed in vacuo. The crude residue was purified by flash chromatography on a 40 g silica gel cartridge using 1:1 ethyl acetate:h...
Embodiment 2
[0302] Preparation of tert-butyl 4-(1H-indol-4-yl)-piperazine-1-carboxylate (A)
[0303]
[0304] Synthesis of 4-piperazin-1-yl-1H-indole
[0305] To a 1000 mL round bottom flask purged and maintained with an inert atmosphere of nitrogen was added a solution of 1H-indol-4-ylamine (2.8 g, 21.05 mmol, 1.00 equiv) in i-PrOH (800 mL). To this was added bis(2-chloroethyl)amine hydrochloride (4.5 g, 25.21 mmol, 1.20 equiv). To this mixture was added Na 2 CO 3 (8.9 g, 83.96 mmol, 4.00 equiv). The resulting reaction solution was allowed to react overnight with stirring while maintaining its temperature at reflux on an oil bath. filter. The filtrate was concentrated by evaporation on a rotary evaporator under vacuum. 4.3 g of 4-piperazin-1-yl-1H-indole (crude) are thus obtained in the form of a red oil.
[0306] Synthesis of tert-butyl 4-(1H-indol-4-yl)-piperazine-1-carboxylate
[0307] To a 1000 mL round bottom flask was added a solution of 4-piperazin-1-yl-1H-indole ...
Embodiment 3
[0310] 4-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-yl)-1H-indazole (B) and 4-(1-methyl-piperidin-4-yl)- 1H- Synthesis of Indazole (C)
[0311]
[0312] Synthesis of 1-methyl-1,2,3,6-tetrahydro-pyridin-4-yl trifluoro-acetate (D)
[0313]
[0314]To a 250 mL 3-neck round bottom flask purged and maintained with an inert atmosphere of nitrogen was added a solution of BuLi (8.5 mL, 2.5M / L, 21.25 mmol, 1.20 equiv) in THF (20 mL). Its temperature was cooled to -78°C. Then a solution of diisopropylamine (2.14 g, 21.15 mmol, 1.20 equiv) in THF (20 mL) was added dropwise thereto under stirring while cooling it to a temperature of -78°C. The resulting solution was allowed to react at -78°C for 30 minutes with stirring. Then a solution of 1-methylpiperidin-4-one (2 g, 17.67 mmol, 1.00 equiv) in THF (32 mL) was added dropwise thereto with stirring while cooling it to a temperature of -78°C. The resulting solution was allowed to react at -78°C for 120 minutes with stirring. C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com