Synthesis method of four-functional group epoxy resin and prepared epoxy resin
A technology of agglomerated epoxy resin and synthesis method, applied in organic chemistry and other directions, can solve the problems of affecting performance and use, low epoxy value of epoxy resin, etc., to improve recovery rate, increase epoxy value, and reduce waste water production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
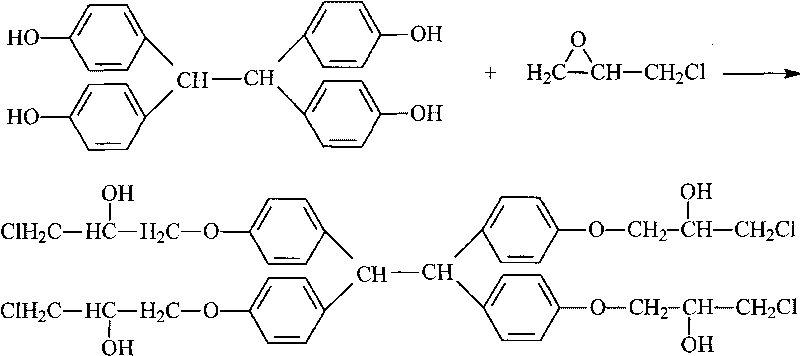
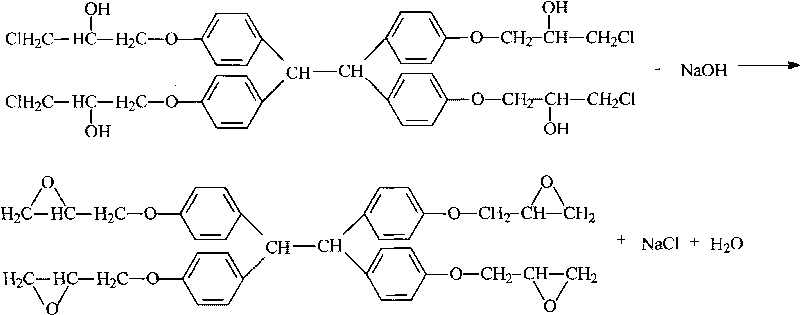
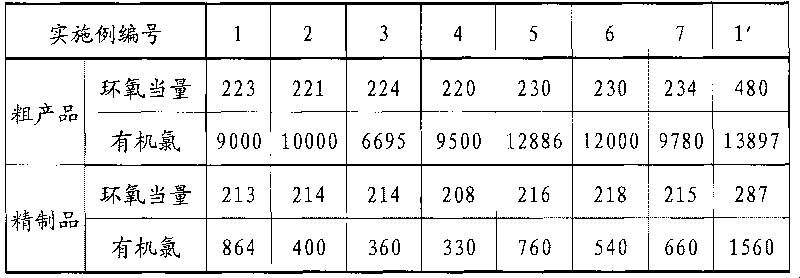
[0033] Add 199g (grams) of phenolic resin, 955g of epichlorohydrin, and 1.0g of tetraethylammonium bromide into a four-necked flask with a volume of 2000mL (milliliters) equipped with a stirring, heating and condensing device, and raise the temperature to 105-110°C , Reflux reaction for 4h for etherification reaction. After the reaction was completed, the temperature was lowered to 40°C, and 174 g of a metered sodium hydroxide aqueous solution with a mass fraction of 48.36% was added dropwise within 2.5 hours to carry out the ring-closing reaction, and the alkali was added to complete the heat preservation reaction for 4 hours. After the reaction is over, use dilute phosphoric acid solution to neutralize to a pH of 7.0-7.5 under stirring conditions, then stand still to separate the liquid to remove the water layer, and the resin layer is decompressed to recover epichlorohydrin and a small amount of water. The conditions for vacuum distillation are: The temperature is 140°C, th...
Embodiment 2
[0035] Synthetic condition is the same as embodiment 1, but adopts to blow into CO in neutralization step 2 to complete neutralization.
Embodiment 3
[0037] The synthesis conditions are the same as in Example 1, but deionized water is used for washing-layering-standstill-water removal process, and the washing process is carried out at least three times so that the pH of the resin system is close to 7.0-7.5.
[0038] Comparative Example 1'
[0039] The synthesis conditions are the same as in Example 1, but there is no neutralization step, and subsequent steps such as epichlorohydrin removal are directly carried out.
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com