Tetrahydroisoquinoline compounds and preparation method and application thereof
A technology of tetrahydroisoquinoline and compounds, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problems of prolonging the microtubule time and changing the "mechanical instability of microtubule"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
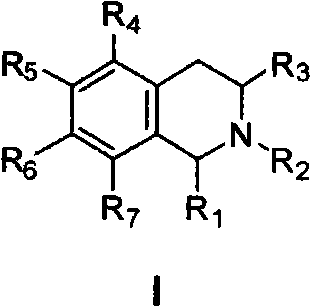
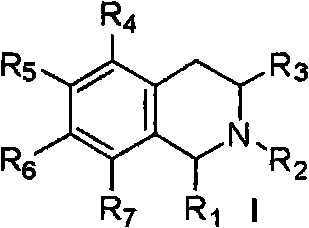
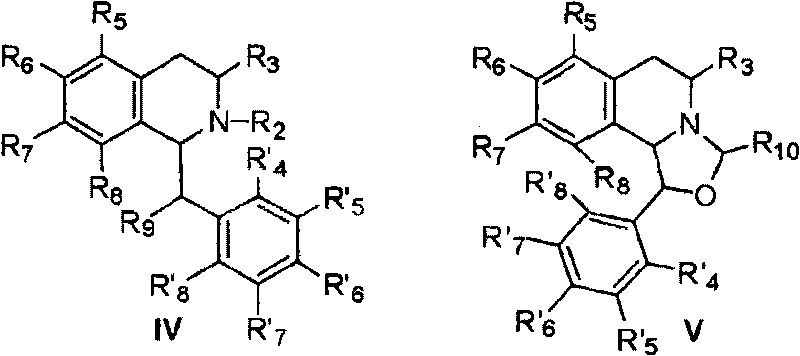
[0059] Example 1: 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-1',3'-dioxole[4',5'-g]-5 Preparation of -isoquinolyl)-1(3H)-isobenzofuranone hydrochloride (1)
[0060] (1) Preparation of N-(4,5-methylenedioxy)phenethyl-4,5-dimethoxy-3-oxo-isobenzofuran-1-amide (104)
[0061] Under argon protection and stirring, a mixture of piperonylethylamine 1.65g (10.0mmol) and dichloromethane 20.0mL was slowly dropped into 4,5-dimethoxy-3-oxoisobenzofuran-1-carboxylate Acid 2.38g (10.0mmol), DCC 2.17g (10.5mmol), DMAP0.12g (1.0mmol) and dichloromethane 40.0mL of the mixed solution. After dropping, react at room temperature for 12.0h. The insoluble matter was filtered off, 50 mL of water was added to the reaction solution, the organic layer was separated, and the aqueous layer was extracted with dichloromethane (30 mL×2). The organic layers were combined, washed successively with 1.0 mol / L hydrochloric acid (50 mL×1), 1.0 mol / L sodium bicarbonate solution (50 mL×1) and saturated brine (50 mL×1), a...
Embodiment 2
[0066] Example 2: 5,6-dimethoxy-3-(5,6,7,8-tetrahydro-1',3'-dioxole[4',5'-g]-5 Preparation of -isoquinolyl)-1(3H)-isobenzofuranone hydrochloride (2)
[0067] The preparation method is the same as that of compound 1, and the yield is 57.6%.
[0068] MS(m / z): 370.1[M+H] + . 1 H NMR (D 2 O) δ2.98~3.02(m, 2H), 3.43~3.47(m, 2H), 3.88(s, 3H), 3.94(s, 3H), 5.35(d, 1H, J=2.4), 5.96(d , 2H, J = 10.9), 6.21 (d, 1H, J = 2.4), 6.29 (s, 1H), 6.68 (s, 1H), 6.85 (s, 1H), 7.37 (s, 1H).
Embodiment 3
[0069] Example 3: 6,7-dimethoxy-3-(6,7-dimethoxy-1,2,3,4-tetrahydro-1-isoquinolinyl)-1(3H)-iso Preparation of Benzofuranone Hydrochloride (3)
[0070] The preparation method is the same as that of compound 1, and the yield is 49.8%.
[0071] MS(m / z): 386.2[M+H] + . 1 H NMR (D 2 O) δ3.00(d, 2H, J=6.0), 3.28(s, 3H), 3.45~3.47(m, 1H), 3.65~3.68(m, 1H), 3.73(s, 3H), 3.74(s , 3H), 3.89(s, 3H), 5.15(s, 1H), 5.72(s, 1H), 6.17(s, 1H), 6.84(s, 1H), 7.28(d, 1H, J=8.4), 7.54 (d, 1H, J = 8.4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com