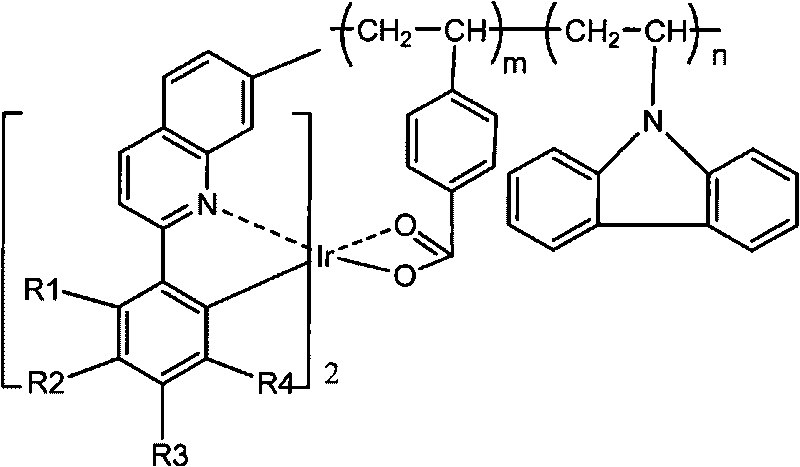
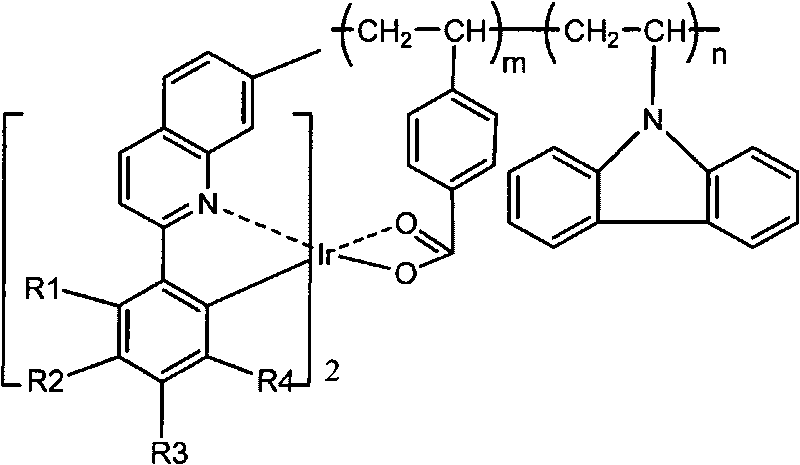
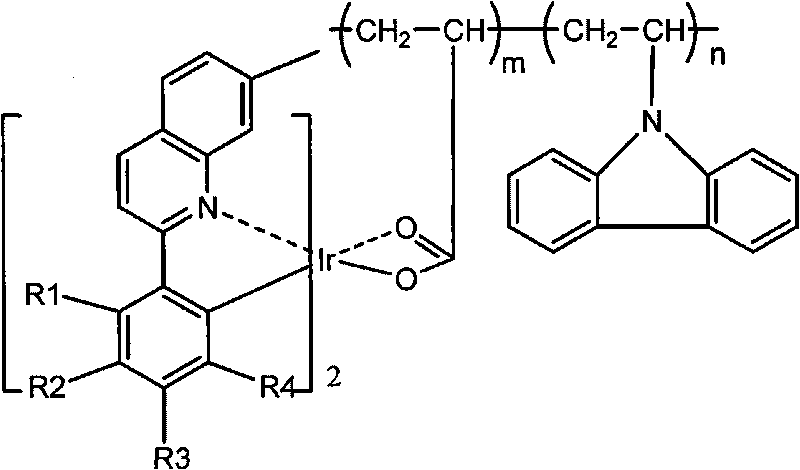
Red phosphorescent macromolecular iridium complex electroluminescent material and preparation method thereof
A red phosphorescence and polymer technology, applied in red phosphorescent polymer iridium complex electroluminescent materials and related preparation fields, can solve the problems of lagging development of red light materials, difficulty in obtaining efficiency and color saturation red light materials, etc. , to achieve the effect of simple preparation method and good processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Add 2.118g (1mmol) iridium chloride trihydrate, 1.05g (4.5mmol) 2-(3-methylphenyl)-7-methylquinoline in a round bottom flask, then add 90mL Ethoxyethanol and 30 mL of distilled water. Heated to 120° C. in a constant temperature oil bath under the protection of nitrogen, and stirred and refluxed for 24 hours. Cool to room temperature, filter with suction, dissolve the product with dichloromethane, and then filter with suction, distill off the dichloromethane in the filtrate, wash the obtained solid with methanol twice, and dry it under vacuum at 60°C for 24 hours to obtain a chlorine-bridged cyclometal iridium dimer. The yield was 46%.
[0037] (2) Add 0.059g (0.4mmol) of p-vinylbenzoic acid, 0.424g (4mmol) of anhydrous sodium carbonate, 0.013g (0.12mmol) of p-benzoquinone, 10mL of tetrahydrofuran and 2mL of distilled water into a round-bottomed flask. After stirring for 3 hours, the reaction was complete, and the solvent was distilled off under reduced pressure. ...
Embodiment 2
[0041] Others are the same as P1, the conjugated unsaturated carboxylic acid ligand is 2-methacrylic acid, and the red phosphorescent polymer iridium complex electroluminescent material P2 is synthesized, and the others are similar to Example 1. The yield was 58%. The number average molecular weight Mn was 4009. The maximum photoluminescence emission wavelength is 610 nm. The structure of P2 is as follows:
[0042]
Embodiment 3
[0044] Others are the same as P1, the conjugated unsaturated carboxylic acid ligand is 2-ethylacrylic acid, and the red phosphorescent polymer iridium complex electroluminescent material P3 is synthesized, and the others are similar to Example 1. The yield was 55%. The number average molecular weight Mn was 3591. The maximum photoluminescence emission wavelength is 610 nm. The P3 structure is as follows:
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com