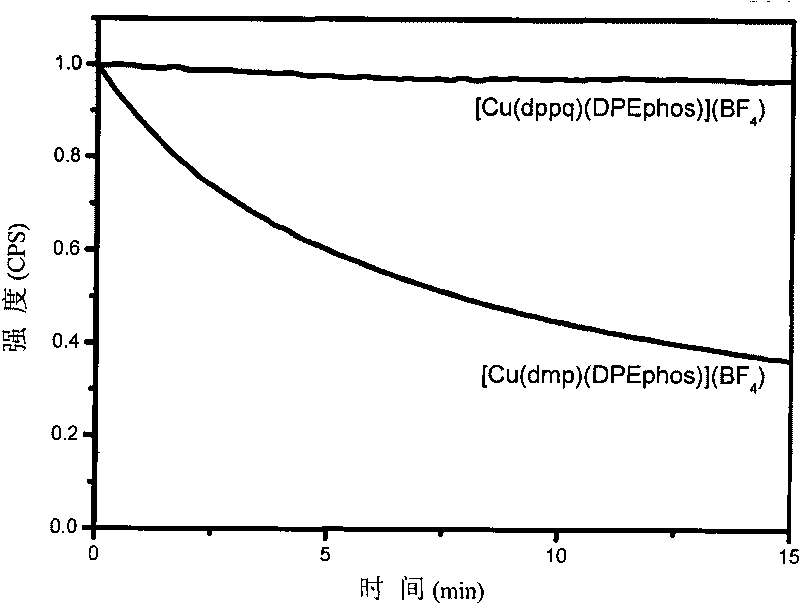
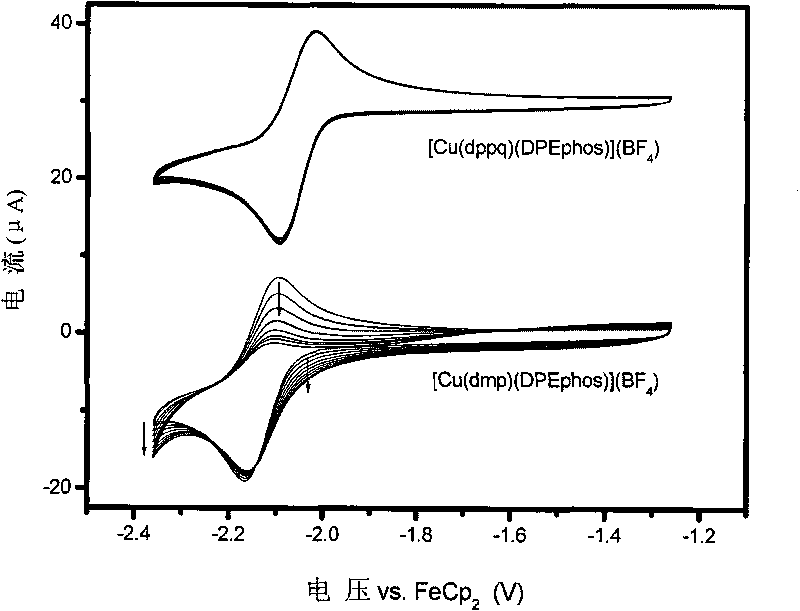
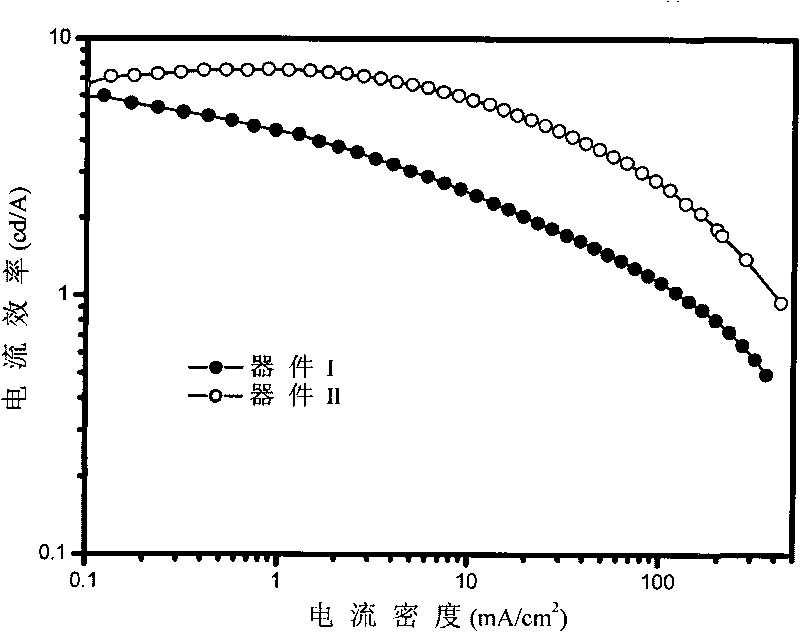
Cooper (I) phosphorescent complexes using 8-phosphinoquinoline derivative as ligand and application thereof
A phosphorescent complex, quinoline technology, applied in the direction of copper organic compounds, phosphorus organic compounds, luminescent materials, etc., can solve the problems of poor stability, photophysical and electrochemical stability to be improved, weakened bond energy and prone to breakage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: [Cu(dppq)(PPh 3 ) 2 ](BF 4 )Synthesis
[0032] Triphenylphosphine (PPh 3 ) (524mg, 2.0mmol) and [Cu(NCCH 3 ) 4 ](BF 4 ) (314mg, 1.0mmol) was dissolved in 10 ml of dichloromethane, stirred at room temperature for 30 minutes, after the solid matter was completely dissolved, then added dppq (313mg, 1.0mmol), continued to stir for 30 minutes, filtered, and the solvent was spin-dried. Dichloromethane / ethanol recrystallization gave yellow crystals [Cu(dppq)(PPh 3 ) 2 ](BF 4 ) 650mg, yield 66%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group P-1, a=12.895(3), b=14.064(3), a=77.905(3), β=83.939(5), γ=89.230(5)°,
[0033]
Embodiment 2
[0034] Example 2: [Cu(mdppq)(PPh 3 ) 2 ](BF 4 )Synthesis
[0035] Triphenylphosphine (PPh 3 ) (524mg, 2.0mmol) and [Cu(NCCH 3 ) 4 ](BF 4) (314 mg, 1.0 mmol) was dissolved in 10 ml of dichloromethane and stirred at room temperature for 30 minutes. Then mdppq (327 mg, 1.0 mmol) was added, stirring was continued for 30 minutes, filtered, and the solvent was spin-dried. Ethanol / ether recrystallization gave yellow crystals [Cu(mdppq)(PPh 3 ) 2 ](BF 4 )392mg, yield 40%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group P2(1) / c, a=10.781(3), b=14.758(3), β=92.910(4),
[0036]
Embodiment 3
[0037] Embodiment 3: [Cu(dppq)(DPEphos)](BF 4 )Synthesis
[0038] 2,2'-bis(diphenylphosphino)diphenyl ether (DPEphos) (538mg, 1.0mmol) and [Cu(NCCH 3 ) 4 ](BF 4 ) (314mg, 1.0mmol) was dissolved in 10 ml of dichloromethane, stirred at room temperature for 30 minutes, after the solid matter was completely dissolved, then added dppq (313mg, 1.0mmol), continued to stir for 30 minutes, filtered, and the solvent was spin-dried. Ethanol / ether recrystallization gave yellow crystals [Cu(dppq)(DPEphos)] (BF 4 )934mg, yield 93%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group P-1, a=11.023(3), b=14.753(4), a=92.285(4), β=91.635(5), γ=96.309(3)°,
[0039]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com