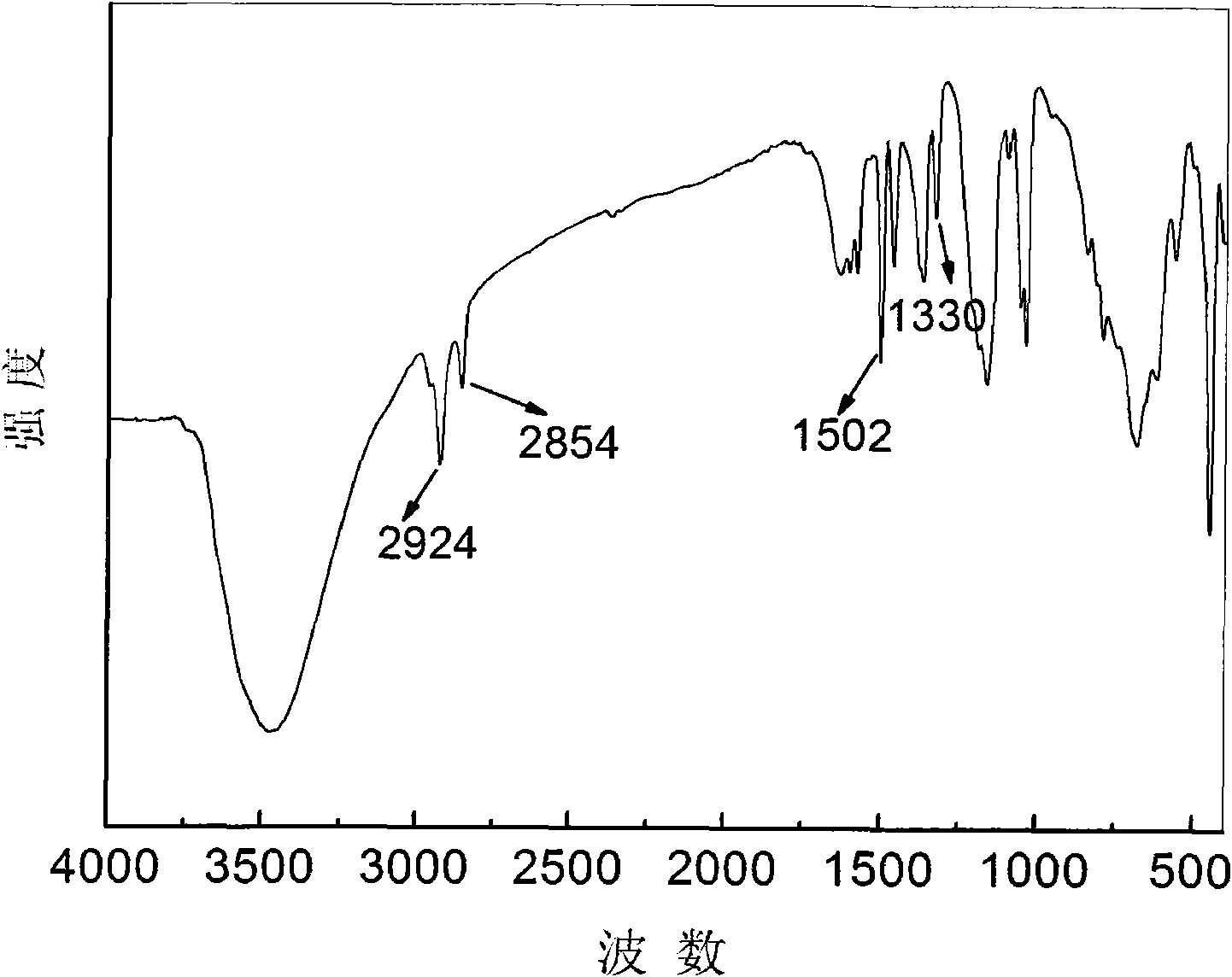
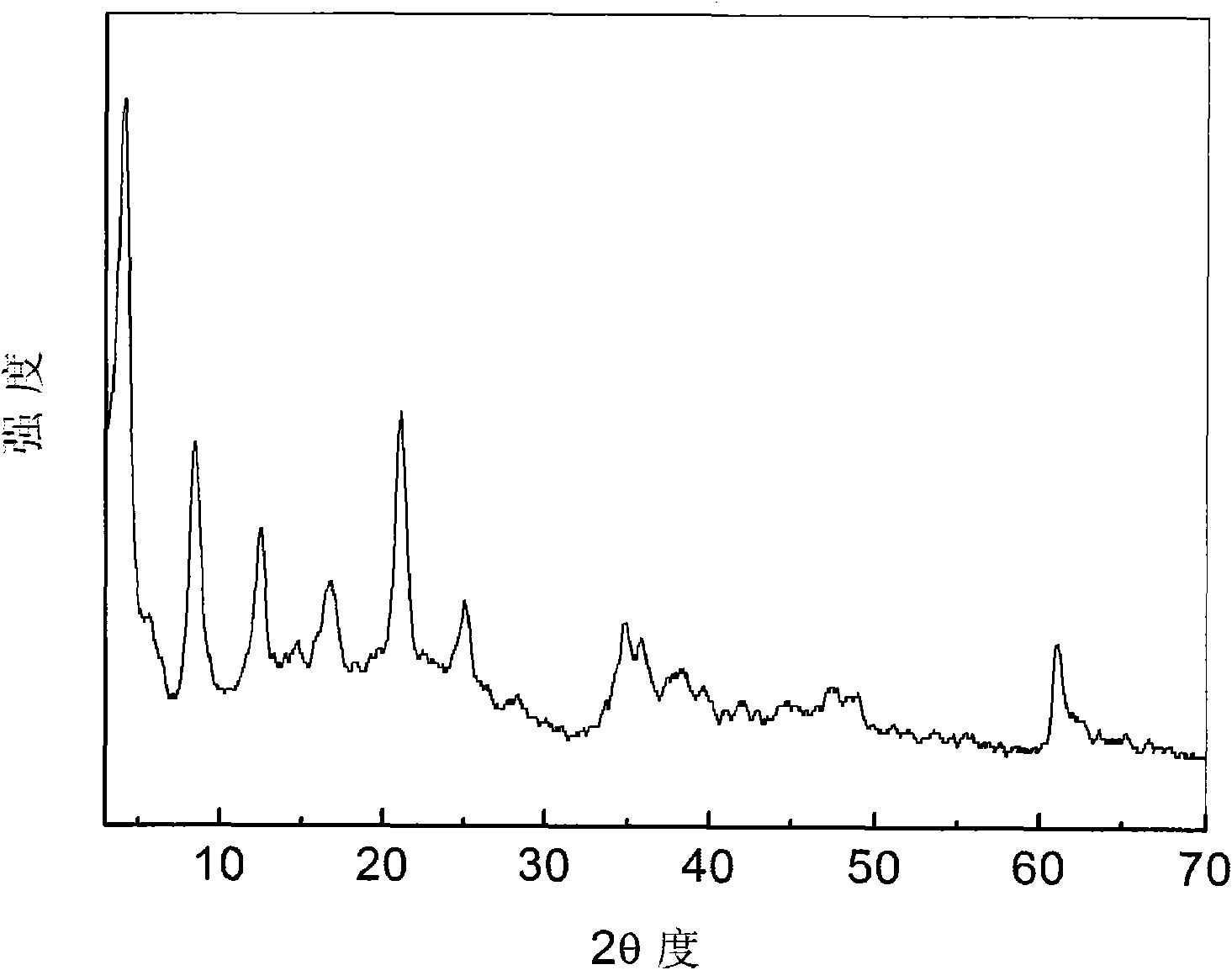
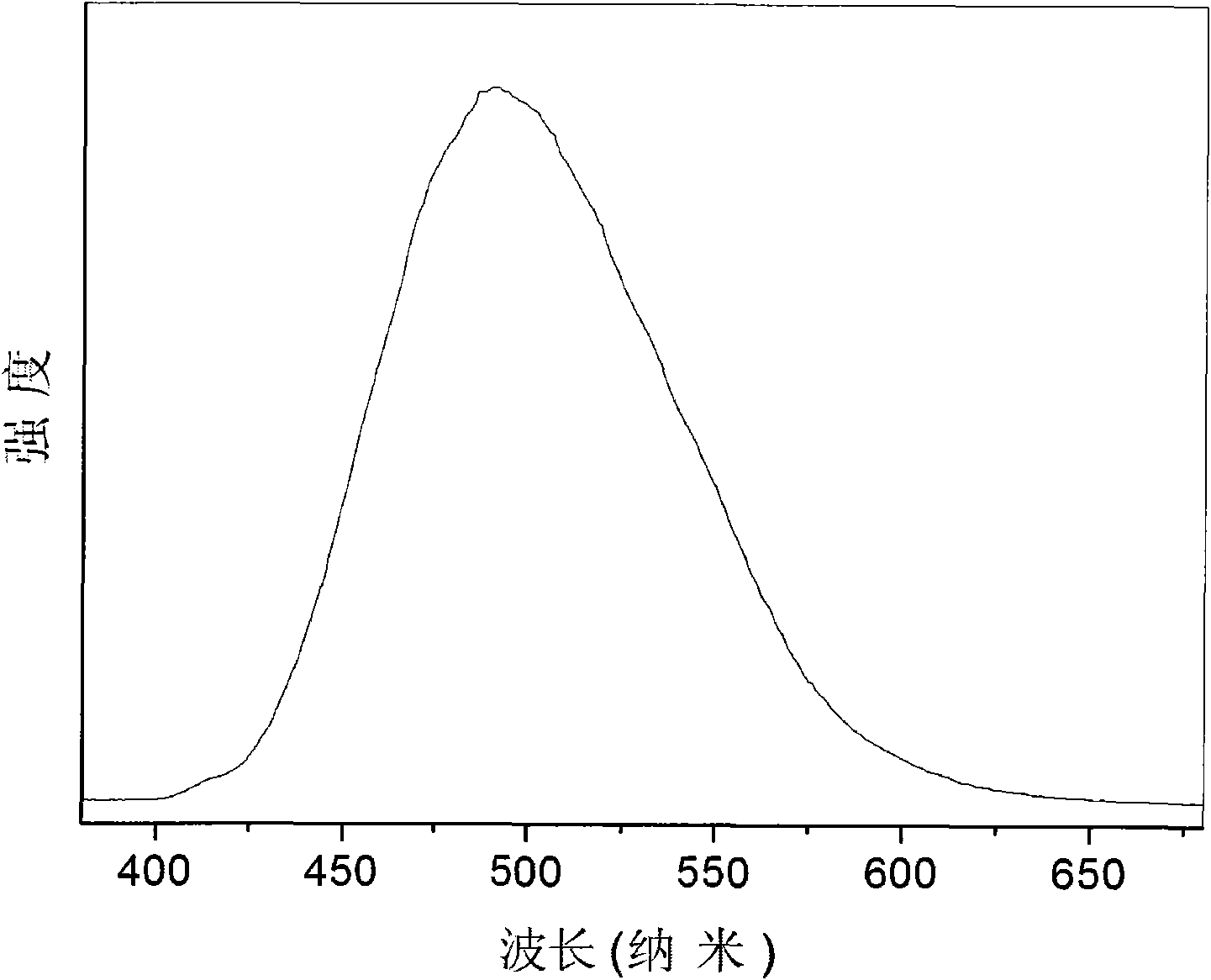
Tri(8-hydroxyquinoline-5-sulfonate) aluminum complex anion intercalated hydrotalcite composite luminescent material and preparation method thereof
An anion intercalation, hydroxyquinoline technology, applied in luminescent materials, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Weigh 0.769g Mg(NO 3 ) 2 ·6H 2 O and 0.563g Al(NO 3 ) 3 9H 2 O dissolved in 50ml to remove CO 2 , deionized water, to obtain solution A, Mg 2+ / Al 3+ The molar ratio is 2:1, Mg 2+ The concentration is 0.06M;
[0035] 2. Dissolve 0.036g of tris(8-hydroxyquinoline-5-sulfonate)aluminum and 0.371g of sodium dodecylsulfonate in 50ml of water to obtain solution B, tris(8-hydroxyquinoline-5- Sodium sulfonate) the concentration of aluminum is 0.0009M;
[0036] 3. Mix solution A and solution B to obtain solution C, and pour solution C into a four-necked flask;
[0037] 4. Dissolve 0.8g NaOH in 40ml to remove CO 2 , deionized water, the NaOH solution was passed through a constant pressure funnel, and under the condition of nitrogen protection, slowly added dropwise to the four-necked flask equipped with solution C until the pH value was 9.5 to obtain slurry D, which was transferred to a 100ml high-pressure The reaction kettle was placed in an oven at 110°C for 20 h...
Embodiment 2
[0041] 1. Weigh 0.769g Mg(NO 3 ) 2 ·6H 2 O and 0.563g Al(NO 3 ) 3 9H 2 O dissolved in 50ml to remove CO 2 , deionized water, to obtain solution A, Mg 2+ / Al 3+ The molar ratio is 2:1, Mg 2+ The concentration is 0.06M;
[0042] 2. Dissolve 0.267g of tris(8-hydroxyquinoline-5-sulfonate)aluminum and 0.136g of sodium dodecylsulfonate in 50ml of water to obtain solution B, tris(8-hydroxyquinoline-5- Sodium sulfonate) the concentration of aluminum is 0.0067M;
[0043] 3. Mix solution A and solution B to obtain solution C, and pour solution C into a four-necked flask;
[0044] 4. Dissolve 0.8g NaOH in 20ml to remove CO 2 , deionized water, the NaOH solution was passed through a constant pressure funnel, and under nitrogen protection conditions, slowly added dropwise to the four-necked flask equipped with solution C until the pH value was 10 to obtain slurry D, which was transferred to a 100ml high-pressure The reaction kettle was placed in an oven at 130°C for 24 hours; ...
Embodiment 3
[0048] 1. Weigh 1.154g Mg(NO 3 ) 2 ·6H 2 O and 0.563g Al(NO 3 ) 3 9H 2 O dissolved in 50ml to remove CO 2 , deionized water, to obtain solution A, Mg 2+ / Al 3+ The molar ratio is 3:1, Mg 2+ The concentration is 0.09M;
[0049] 2. Dissolve 0.392g of tris(8-hydroxyquinoline-5-sulfonate)aluminum and 0.008g of sodium dodecylsulfonate in 50ml of water to obtain solution B, tris(8-hydroxyquinoline-5- Sodium sulfonate) the concentration of aluminum is 0.0098M;
[0050] 3. Mix solution A and solution B to obtain solution C, and pour solution C into a four-necked flask;
[0051] 4. Dissolve 0.8g NaOH in 40ml to remove CO 2 , deionized water, the NaOH solution was passed through a constant pressure funnel, and under nitrogen protection conditions, slowly added dropwise to the four-necked flask equipped with solution C until the pH value was 11 to obtain slurry D, which was transferred to a 100ml high-pressure The reaction kettle was placed in an oven at 140°C for 36 hours; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com