A novel bone mass increasing agent
A bone mass and drug technology, applied in the field of enhancing bone formation, can solve the problem of unexplained mechanism and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1: Differentiation of human mesenchymal stem cells
[0155] Reagent
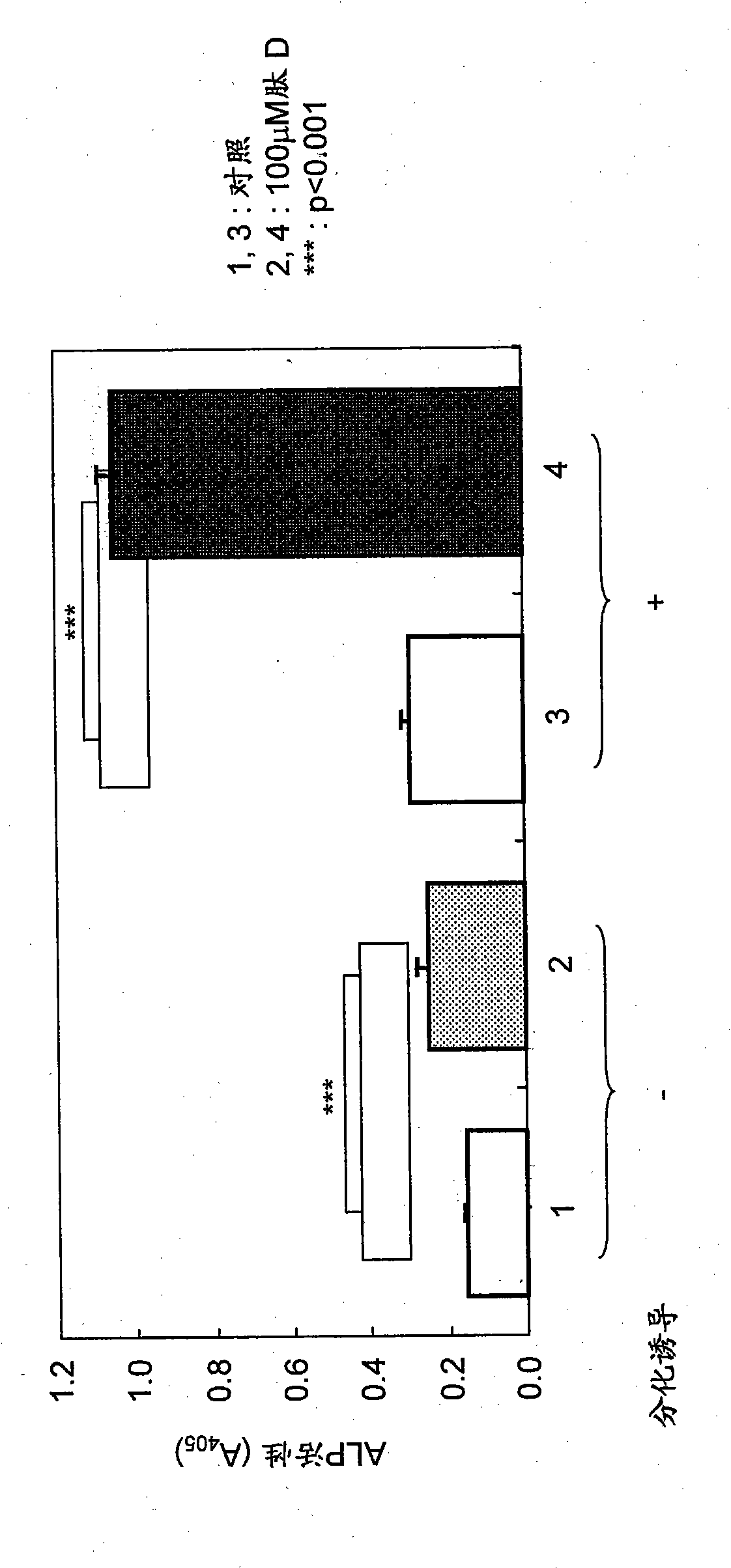
[0156] Synthetic peptides were used as experimental reagents. Synthetic peptide D is a peptide having the amino acid sequence shown in SEQ ID NO: 7, and is a cyclic peptide consisting of 9 amino acids and containing 2 cysteine residues bonded to each other by a disulfide bond. Synthetic peptide D has been reported to bind to RANKL (Aoki et al., JClin Invest 116:1525, 2006). As a control peptide, a synthetic peptide lacking the above-mentioned functions was used.
[0157] Cultured cells
[0158] Human mesenchymal stem cells were purchased from Cambrex Corporation. Maintenance medium produced by Lonza was used for subculture.
[0159] Differentiation of human mesenchymal stem cells
[0160] Human mesenchymal stem cells were seeded into 96-well plates (1×10 3 cells / well) (Nunc) and 48-well plates (2.4×10 3 cells / well) (IWAKI). After 24 hours, the culture supernatant was removed from ea...
Embodiment 2
[0165] Example 2: Calcification of human mesenchymal stem cells
[0166] ALP staining
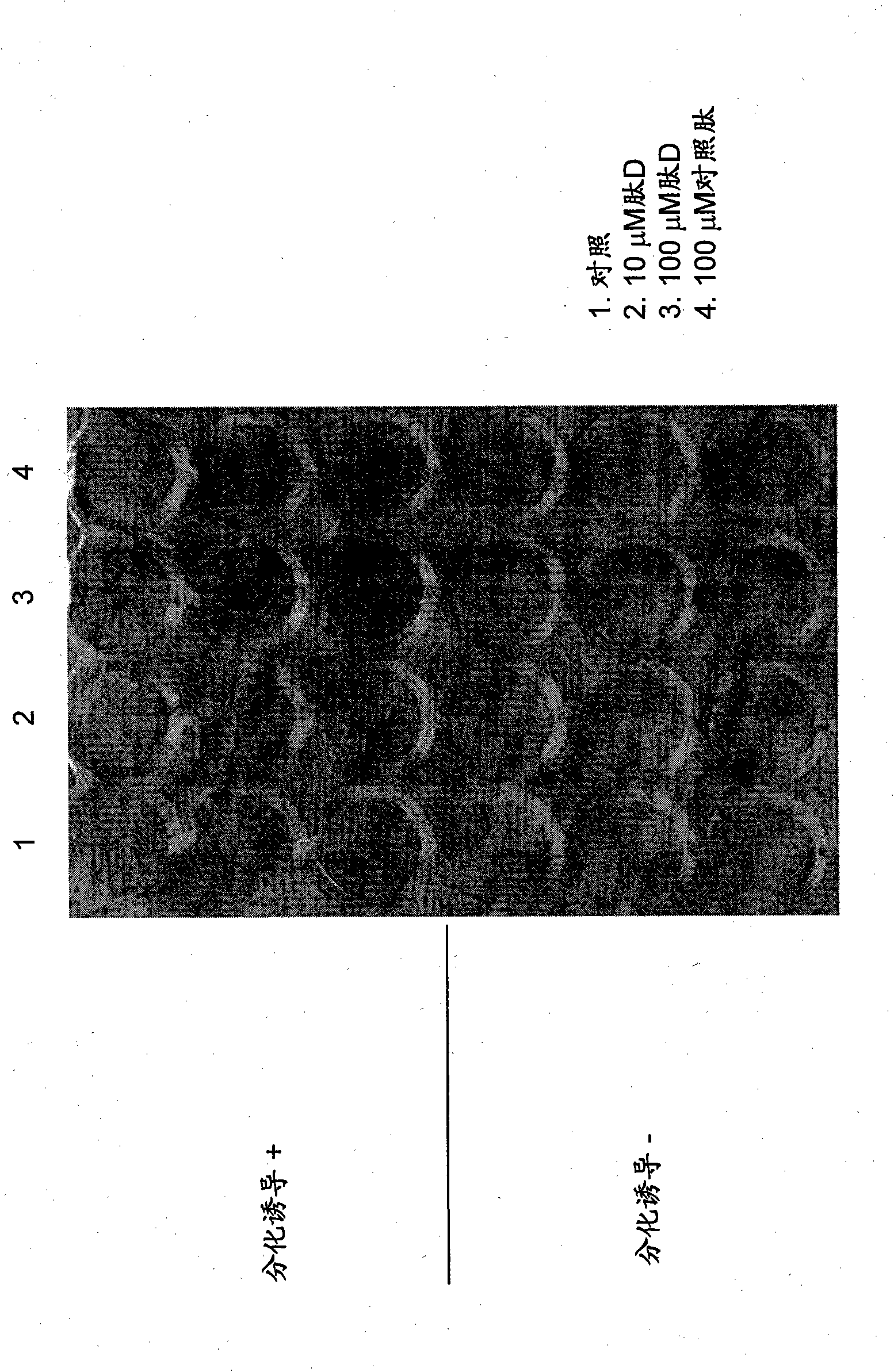
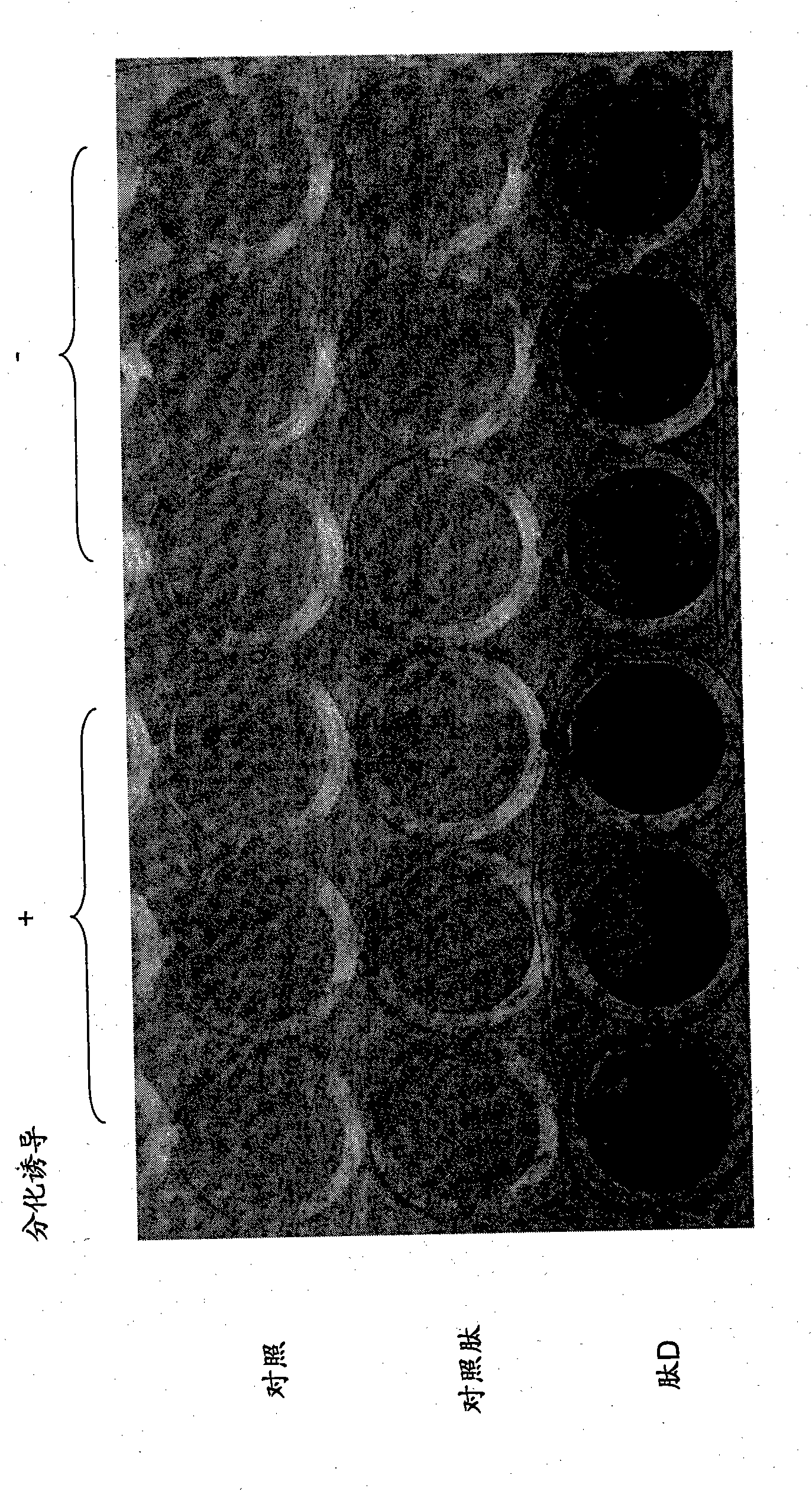
[0167] During differentiation, peptide D was added at a concentration of 300 μM. On day 7, cells were fixed with 10% neutral buffered formalin solution, followed by refixation with acetone / ethanol solution.
[0168] A staining solution (500 µL) prepared according to the following composition was added to the cells, which were further incubated at 37°C for 10 minutes, washed with water, and dried.
[0169] (composition of staining solution)
[0170] Naphthol AS-MX Phosphate (SIGMA): 5mg
[0171] N,N-Dimethylformamide (Wako): 0.5 mL
[0172] 0.1M Tris-HCl (pH 8.5): 50mL
[0173] Fast blue half salt (SIGMA): 30mg
[0174] Alizarin red S staining
[0175] On day 21 after differentiation, the cells were washed with PBS and fixed with 10% neutral buffered formalin solution.
[0176] After removing the fixative, the cells were washed with water. 1% Alizarin Red S staining solution (Nacala...
Embodiment 3
[0178] Example 3: Differentiation of mouse osteoblast precursor cell line (MC3T3-E1)
[0179] Cultured cells
[0180] Mouse osteoblast precursor cell line MC3T3-E1 (subclone number 4) cells were purchased from ATCC.
[0181] Mouse osteoblast precursor cells (MC3T3-E1)
[0182] Cells mixed with 10% FBS+αMEM (GIBCO) were seeded into 96-well plates (8×10 3 cells / well) and 48-well plate (2×10 4 cells / well). After 48 hours, the culture supernatant of each plate was replaced with 10% FBS+αMEM containing 5 mM β-glycerophosphate (SIGMA) and 10 μg / mL sodium ascorbate (SIGMA). Then, replace with new medium every 3 or 4 days. As a control, MC3T3-E1 was cultured using 10% FBS+αMEM as a maintenance medium. Once the medium was changed, peptides were added to the plates at a concentration of 300 [mu]M. According to the method described in Example 1, ALP activity measurement and Alizarin Red S staining were performed on the 7th day and the 21st day.
[0183] Differentiation of mouse o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com