Chewable tablet containing montelukast sodium
A technology of montelukast sodium and chewable tablets, which is applied in the field of chewable tablets containing montelukast sodium, which can solve the problems of no mention of other effects of coloring agents, the easy discoloration of montelukast sodium when exposed to light, and the inconvenience of mass production in the workshop and other problems, to avoid photodecomposition, improve operability, and facilitate production operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Montelukast sodium chewable tablets
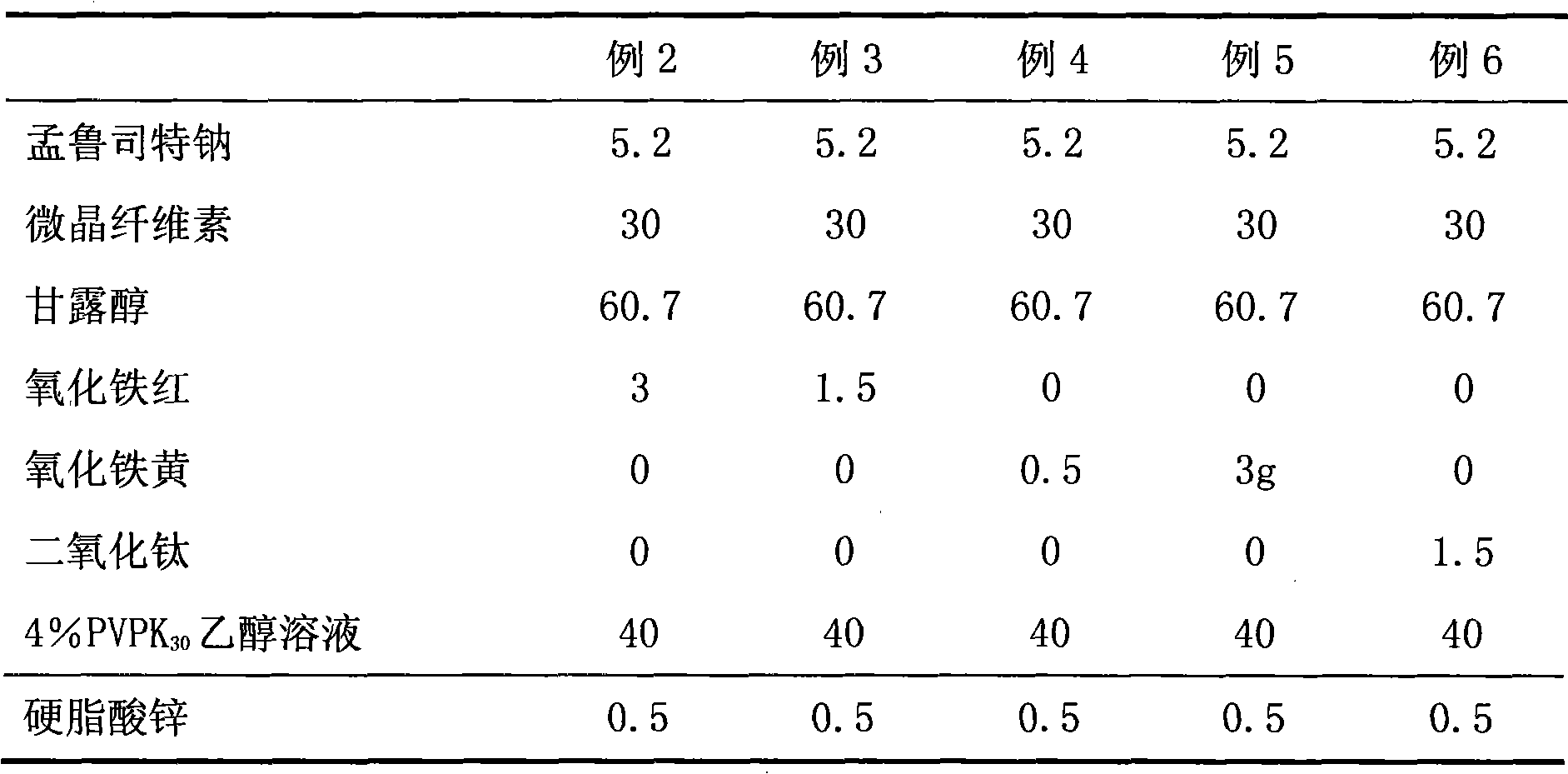
[0031] Montelukast Sodium 5.2g
[0032] Microcrystalline cellulose 30g
[0033] Mannitol 60.7g
[0034] Iron oxide red 1.5
[0035] 4% PVPK30 ethanol solution 40g
[0037] Preparation process: Pass the montelukast sodium and iron oxide red through a 120-mesh sieve, microcrystalline cellulose and mannitol through a 100-mesh sieve, and then mix the iron oxide red and the montelukast sodium by equal volume addition. After mixing, pass through a 100-mesh sieve twice, and then mix the above-mentioned mixed powder with mannitol in equal increments, and pass through an 80-mesh sieve after each mixing, and then add all the added raw and auxiliary materials to 4% PVPK 30 Alcohol solution is granulated, and after drying, the prescription amount of zinc stearate is added and mixed uniformly, and the tablet is obtained.
Embodiment 2-6
[0038] Example 2-6 Montelukast Sodium Chewable Tablets
[0039] For the convenience of description, Table 1 describes Examples 2-6 below. The preparation process of Examples 2-6 is the same as that of Example 1.
[0040] Table 1 Example 2-6 Montelukast Sodium Chewable Tablets Process Formula
[0041]
[0042] Note: "Example 2" in the table indicates the content of each cube corresponding to Example 2, in g; Example 3 to Example 6 and so on.
Embodiment 7-12
[0043] Example 7-12 Montelukast Sodium Chewable Tablets
[0044] For the convenience of description, Table 2 describes Examples 7-12 below. The preparation process of Examples 7-12 is the same as that of Example 1.
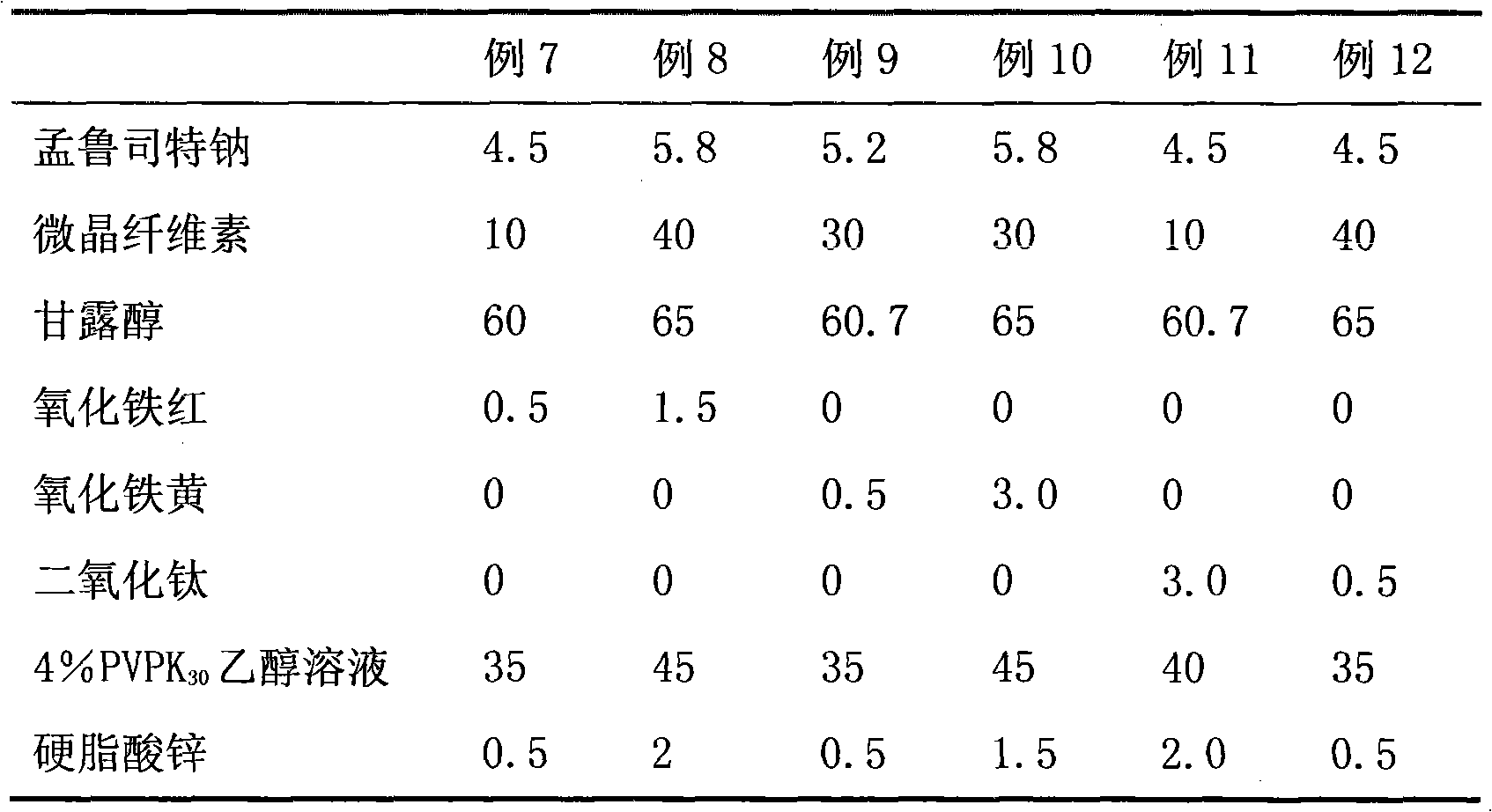
[0045] Table 2 Example 7-12 Montelukast Sodium Chewable Tablet Process Formula
[0046]
[0047] Note: "Example 7" in the table indicates the content of each cube corresponding to Example 7, in g; Example 8 to Example 12 and so on.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com