Method for preparing silicon tetrafluoride by quartz sand
A technology of silicon tetrafluoride and quartz sand, applied in the directions of halogenated silicon compounds, halogenated silanes, etc., can solve the problems of low SiF4 yield, increased production cost, low device output, etc., to overcome high cost and reduced energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
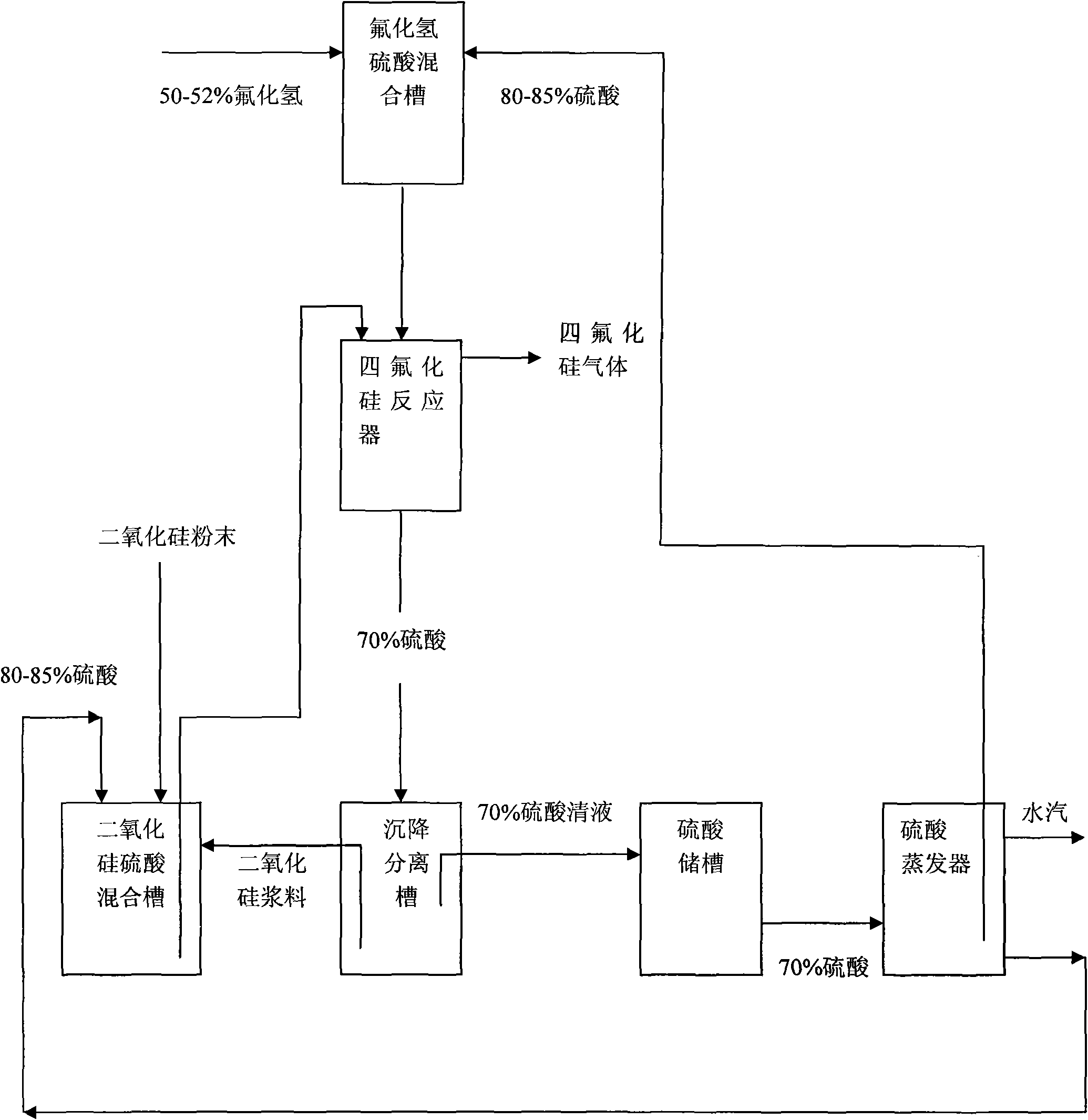
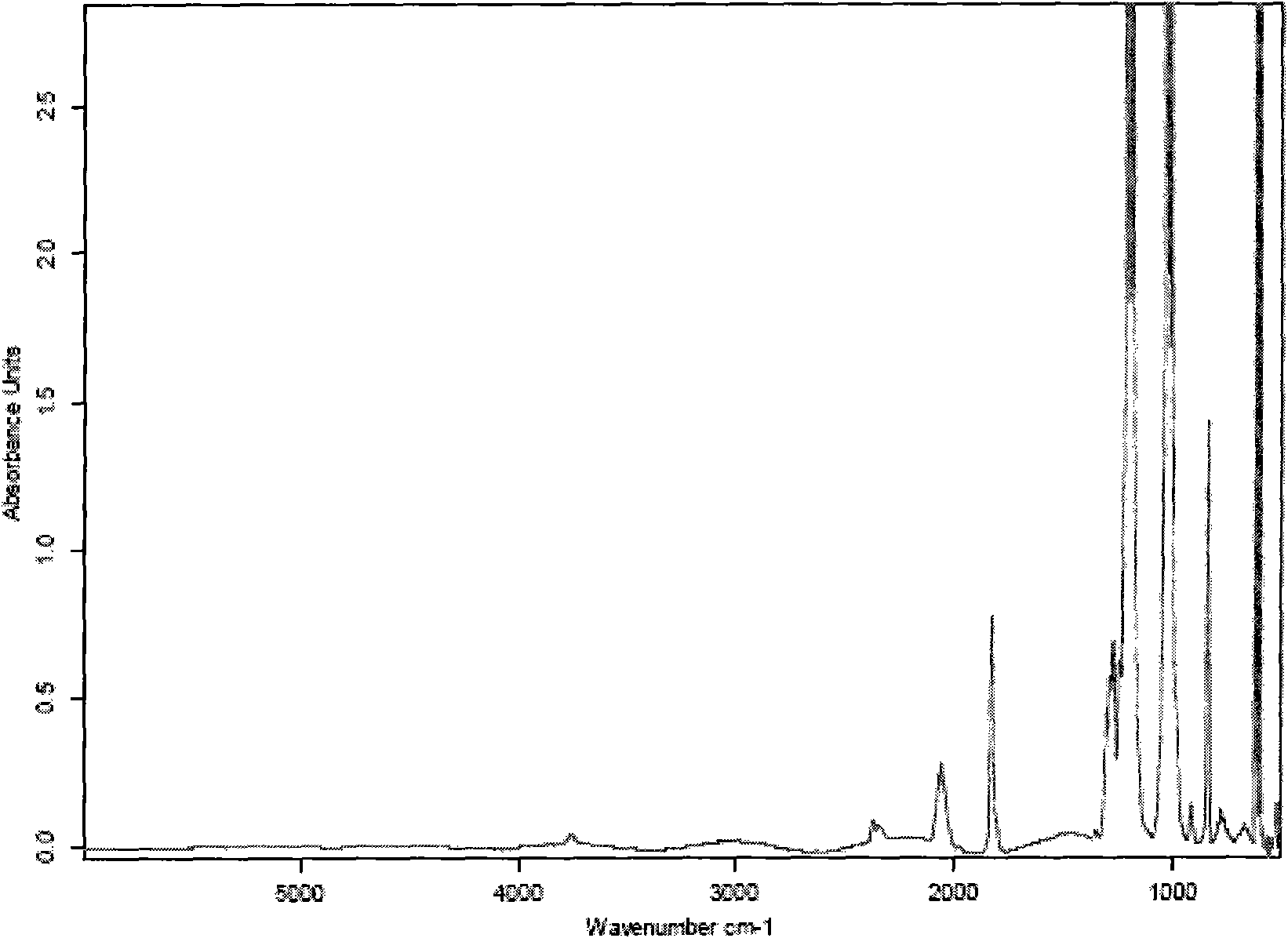
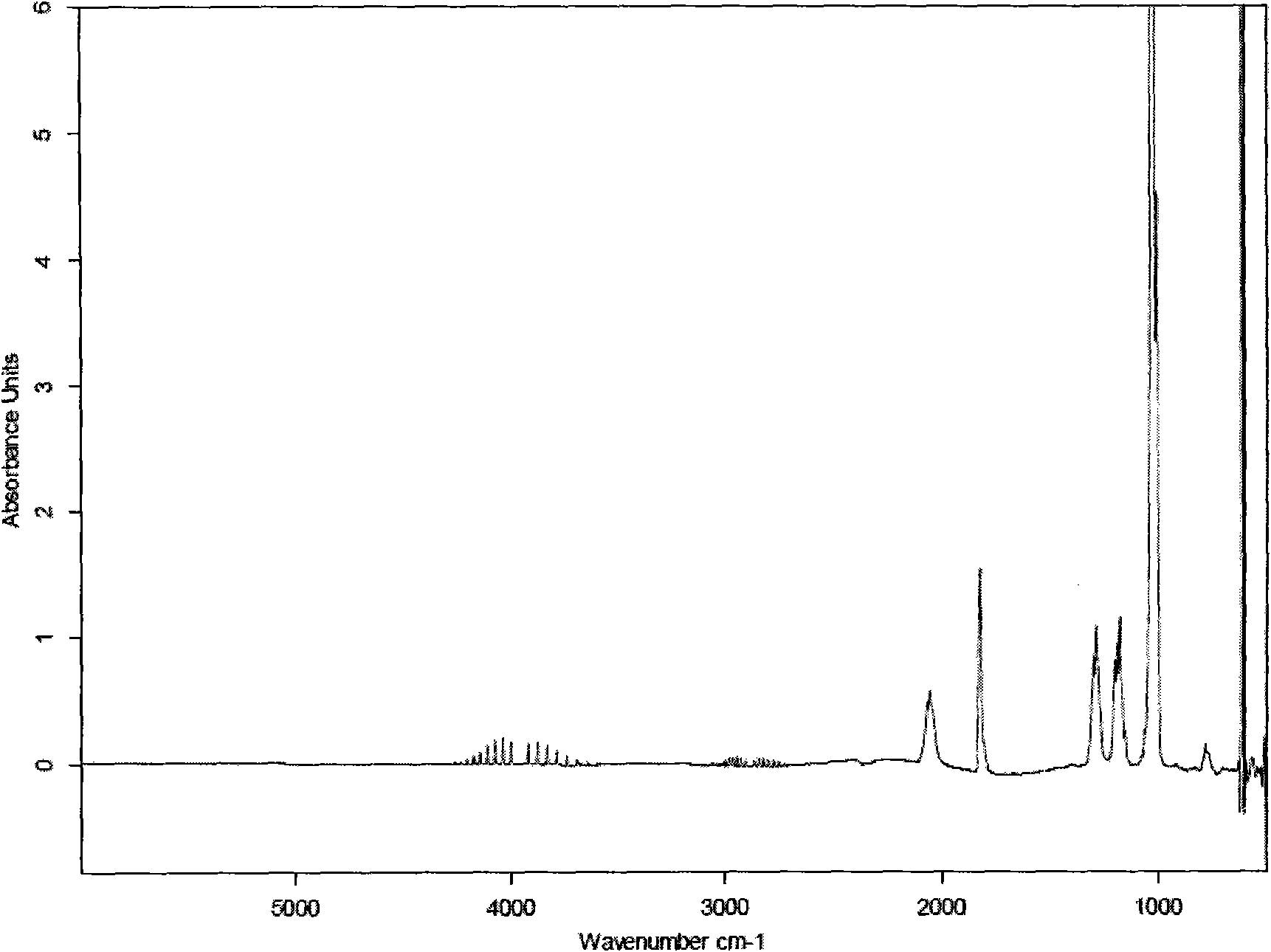
[0049] Get 200 parts by weight of 150-200 mesh silicon dioxide powder, 1000 parts by weight of sulfuric acid with a concentration of 96% by mass, 500 parts by weight of sulfuric acid with a concentration of 80% by mass and 560 parts by weight of hydrofluoric acid with a concentration of 50% by mass Standby; get 1000 parts by weight of sulfuric acid with a mass percentage concentration of 96% and 560 parts by weight of hydrofluoric acid with a mass percentage concentration of 50% for premixed absorption. After the premixed absorption, the concentration of hydrofluoric acid is 12%, and the concentration of sulfuric acid 80% to obtain mixture A; take 200 parts by weight of 150-200 mesh silica powder and 500 parts by weight of sulfuric acid with a mass percentage concentration of 80% for pre-mixing to obtain mixture B; add mixture A and mixture B to the reaction In the reactor, stir the reaction, the reaction time is 10 minutes, the reaction temperature is 60°C, and silicon tetrafl...
Embodiment 2
[0052] Get 220 parts by weight of 150-200 mesh silicon dioxide powder, 1100 parts by weight of sulfuric acid with a concentration of 98% by mass, 550 parts by weight of sulfuric acid with a concentration of 85% by mass and 575 parts by weight of hydrofluoric acid with a concentration of 52% by mass Standby; get 1100 parts by weight of sulfuric acid with a mass percentage concentration of 98% and 575 parts by weight of hydrofluoric acid with a mass percentage concentration of 52% for premixed absorption. After the premixed absorption, the concentration of hydrofluoric acid is 24%, and the concentration of sulfuric acid 85% to obtain mixture A; take 220 parts by weight of 150-200 mesh silica powder and 550 parts by weight of sulfuric acid with a concentration of 85% by mass to pre-mix to obtain mixture B; add mixture A and mixture B to the reaction Stir the reaction in a container, the reaction time is 120 minutes, the reaction temperature is 80°C, and silicon tetrafluoride gas is ...
Embodiment 3
[0055] Get 210 parts by weight of 150-200 mesh silicon dioxide powder, 1050 parts by weight of sulfuric acid with a concentration of 97%, 385 parts by weight of sulfuric acid with a concentration of 82% and 568 parts by weight of hydrofluoric acid with a concentration of 51%. Standby; get 1050 parts by weight of sulfuric acid with a mass percentage concentration of 97% and 568 parts by weight of hydrofluoric acid with a mass percentage concentration of 51% for premixed absorption. After the premixed absorption, the concentration of hydrofluoric acid is 15%, and the concentration of sulfuric acid 82% to obtain mixture A; take 210 parts by weight of 150-200 mesh silica powder and 385 parts by weight of sulfuric acid with a mass percentage concentration of 82% to pre-mix to obtain mixture B; add mixture A and mixture B to the reaction In the container, stir the reaction, the reaction time is 120 minutes, the reaction temperature is 70°C, and silicon tetrafluoride gas is obtained. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com