Recombinant expression of human TIM-1-Fc fusion protein
A fusion protein, human immunoglobulin technology, applied in the field of recombinant expression of TIM-1Fc, can solve the problems of affecting protein activity, unable to meet the activity, and low product recovery rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Artificial synthesis of Tim-1 gene
[0063] According to the human Tim-1 gene sequence searched in the gene bank, the human Tim-1 gene was artificially synthesized, and the mouse IgK secretion signal was added to its N-terminus (5' end), according to the degeneracy of codons Eliminate the enzyme cutting site used in this experiment; the synthesized Tim-1 was subcloned into the mammalian cell expression vector pcDNA3.1 vector, and the vector plasmid pcDNA3.1-Tim containing the Tim-1 gene was amplified and extracted -1.
[0064] The sequence of the mouse IgK secretion signal-Tim-1 is as follows (SEQ ID NO: 1; the underlined part in italics indicates the mouse IgK secretion signal):
[0065] TCTGTAAAGGTTGGTGGAGAGGCAGGTCCATCTGTCACACTACCCTGCCACTACAGTGGAGCTGTCACATCCATGTGCTGGAATAGAGGCTCATGTTCTCTATTCACATGCCAAAATGGCATTGTCTGGACCAATGGAACCCACGTCACCTATCGGAAGGACACACGCTATAAGCTATTGGGGGACCTTTCAAGAAGGGATGTCTCTTTGACCATAGAAAATACAGCTGTGTCTGACAGTGGCGTATATTGTTGCCGTGTTGAGCACCGTG...
Embodiment 2
[0066] Example 2 Construction of pcDNA3.1-Tim-1-Fc expression vector
[0067] The Fc fragment (hinge-CH2-CH3) of human IgG1 was fished from the human cDNA library, and the human Fc fragment (hinge-CH2-CH3) was cloned into pcDNA3.1-Tim-1, and the accuracy of the constructed plasmid was verified by sequencing; Extract the kit, and extract the recombinant plasmid DNA, pcDNA3.1-Tim-1-Fc, according to the instructions.
[0068] The Fc fragment sequence of human IgG1 is as follows (SEQ ID NO: 4):
[0069]GCCTCCACCAAGGGCCCATCGGTCTTCCCCCTGGCACCCTCCTCCAAGAGCACCTCTGGGGGCACAGCGGCCCTGGGCTGCCTGGTCAAGGACTACTTCCCCGAACCGGTGACGGTGTCGTGGAACTCAGGCGCCCTGACCAGCGGCGTGCACACCTTCCCGGCTGTCCTACAGTCCTCAGGACTCTACTCCCTCAGCAGCGTGGTGACCGTGCCCTCCAGCAGCTTGGGCACCCAGACCTACATCTGCAACGTGAATCACAAGCCCAGCAACACCAAGGTGGACAAGAAAGTTGAGCCCAAATCTTGTGACAAAACTCACACATGCCCACCGTGCCCAGCACCTGAACTCCTGGGGGGACCGTCAGTCTTCCTCTTCCCCCCAAAACCCAAGGACACCCTCATGATCTCCCGGACCCCTGAGGTCACATGCGTGGTGGTGGACGTGAGCCACGAAGACCCTGAGGTCAAGTTCAACTGGTACGTGG...
Embodiment 3
[0070] Example 3 Obtaining of recombinant Tim-1-Fc protein
[0071] 1. Induced expression
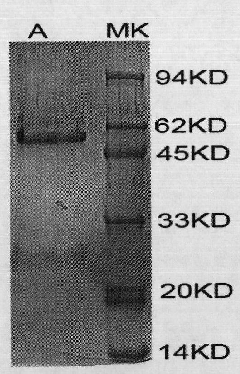
[0072] The sequenced correct pcDNA3.1-Tim-1-Fc was used Lipofectamine TM -2000 was transfected into CHO cells with a fusion rate of 75-85%. After culturing for 48 hours, the cells and their supernatant were collected. Detect the results of each step and control the quality of the final product by SDS-PAGE electrophoresis. Verify the correctness of the target protein by Western-blot ( figure 1 ).
[0073] 2. Protein purification
[0074] According to the above-mentioned optimal conditions for fusion protein expression, expand the culture volume, collect the supernatant, and purify the expressed recombinant protein Tim-1-Fc through various chromatography methods such as affinity, ion exchange, hydrophobicity and gel filtration. The final protein The purity of the product is generally not less than 80%. Detect the results of each step and control the quality of the final product by S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com