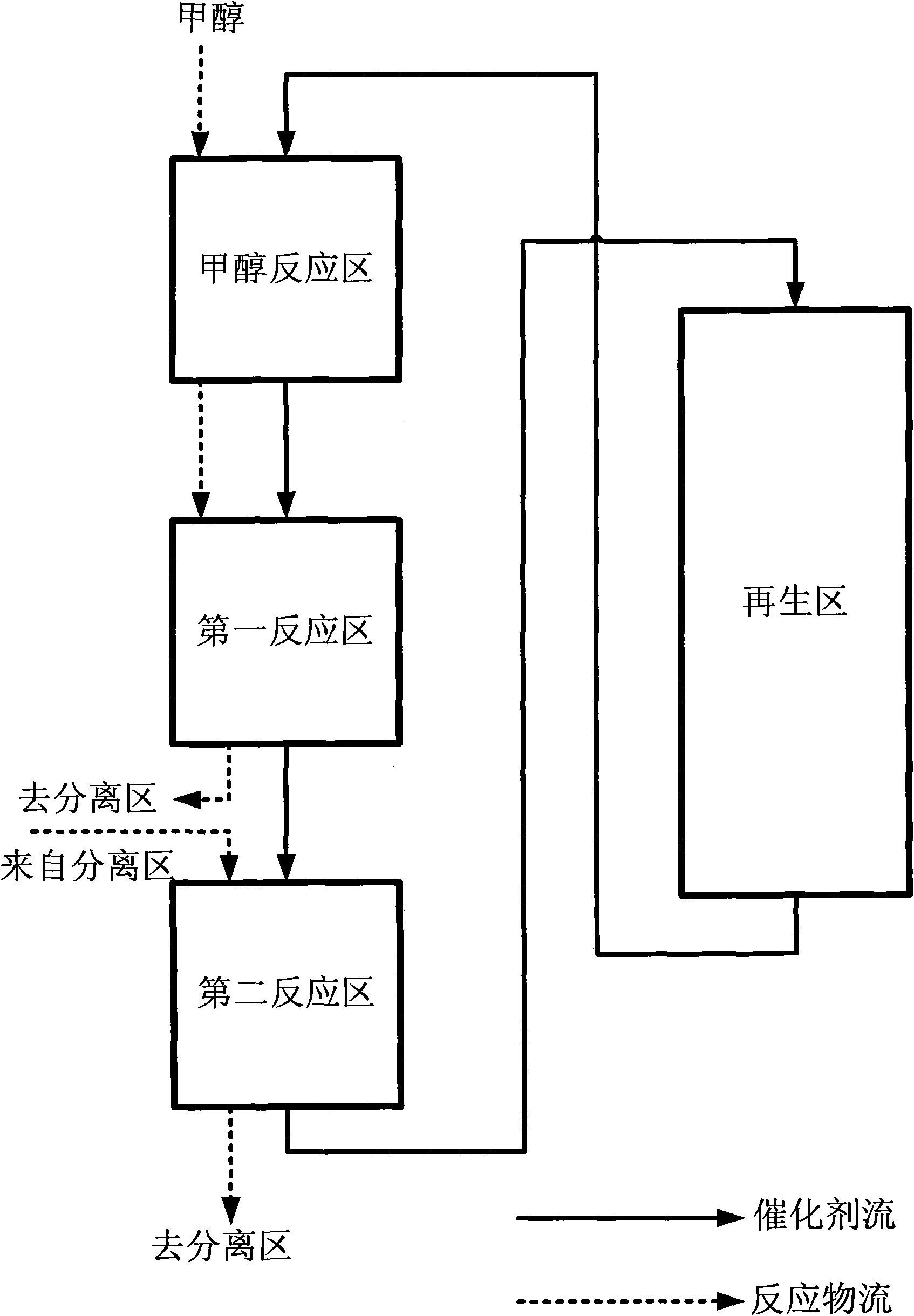
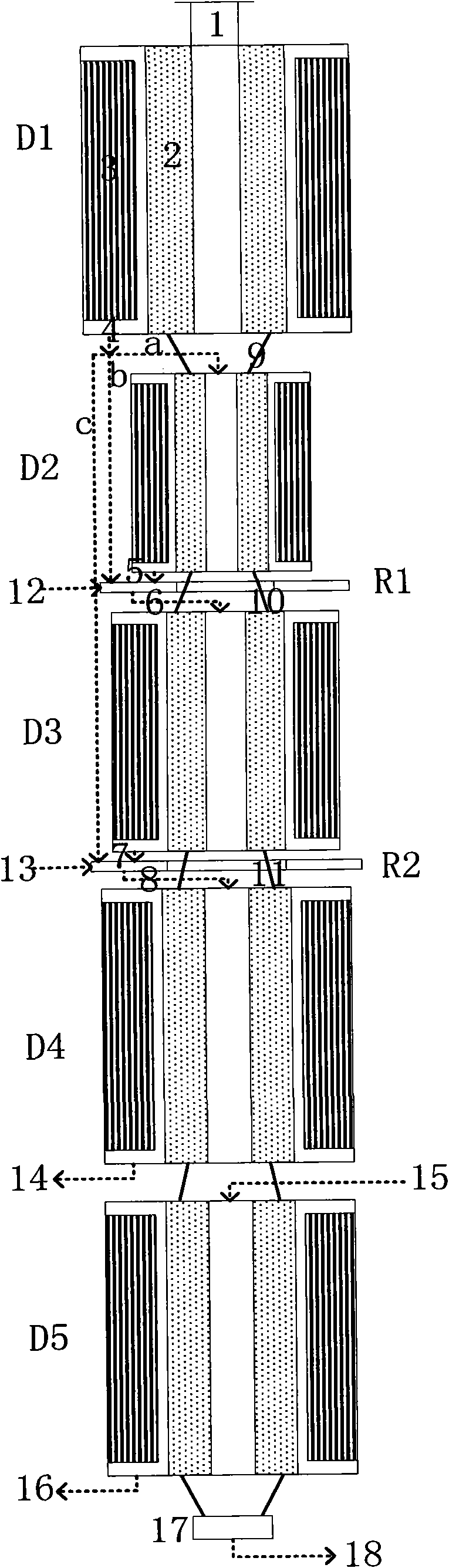
Method for converting methanol into propylene
A technology of methanol conversion and methanol, which is applied in organic chemistry, hydrocarbon cracking, production of bulk chemicals, etc., can solve the problems of low propylene selectivity and discontinuous production, and improve propylene selectivity, increase raw material processing capacity, Achieving a continuous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The catalyst used in this example is a ZSM-5 molecular sieve spherical catalyst with a particle size of 1.6mm-2mm, and the raw material used is methanol.
[0057] The methanol reaction zone adopts a moving bed reactor with an inlet temperature of 250°C and normal pressure operation.
[0058] The first reaction zone adopts three moving bed reactors, each with an inlet temperature of 500°C, operated under normal pressure, and the mixture of methanol, dimethyl ether and water is divided into three reactant streams according to the weight ratio of 1:1.4:1.8; the quenching liquid uses methanol .
[0059] The second reaction zone adopts a moving bed reactor with an inlet temperature of 525°C and normal pressure operation.
[0060] The carbon deposition catalyst carbon deposition amount removed from the bottom of the catalyst bed is less than 2%, and it is sent to the regenerator for regeneration. The catalyst carbon deposition amount after regeneration is less than 0.5% (car...
Embodiment 2
[0071] The catalyst used in this example is a ZSM-5 molecular sieve spherical catalyst with a particle size of 1.6mm-2mm, and the raw material used is methanol.
[0072] Two moving bed reactors are used in the methanol reaction zone, each with an inlet temperature of 280°C and normal pressure operation.
[0073] The first reaction zone adopts four moving bed reactors, each inlet temperature is 450°C, operated under normal pressure, the mixture of methanol, dimethyl ether and water is divided into four reactant streams according to the weight ratio of 1:1.4:1.8:1.2; A mixture of methanol and water was used.
[0074] The second reaction zone adopts a moving bed reactor with an inlet temperature of 465°C and normal pressure operation.
[0075] The carbon deposition catalyst carbon deposition amount removed from the bottom of the catalyst bed is less than 2%, and it is sent to the regenerator for regeneration. The catalyst carbon deposition amount after regeneration is less than ...
Embodiment 3
[0085] The catalyst used in this example is a ZSM-5 molecular sieve spherical catalyst with a particle size of 2mm-3mm, and the raw material used is methanol.
[0086] The methanol reaction zone adopts a moving bed reactor, each inlet temperature is 300°C, and it operates under normal pressure.
[0087] The first reaction zone adopts three moving bed reactors, each inlet temperature is 450°C, and is operated under normal pressure. The mixture of methanol, dimethyl ether and water is divided into three reactant streams according to the weight ratio of 1:1.4:1.8; the quenching liquid uses methanol Mixture with water.
[0088] The second reaction zone adopts a moving bed reactor with an inlet temperature of 480°C and normal pressure operation.
[0089] The carbon deposition catalyst carbon deposition amount removed from the bottom of the catalyst bed is less than 2%, and it is sent to the regenerator for regeneration. The catalyst carbon deposition amount after regeneration is l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com