Antimicrobial gas-releasing ear drainage tubes
An anti-microbial and gas technology, applied in the direction of wound drainage devices, antibacterial drugs, drug combinations, etc., can solve the problems that the embedded structure is not suitable, the effectiveness is not good, and the possibility is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
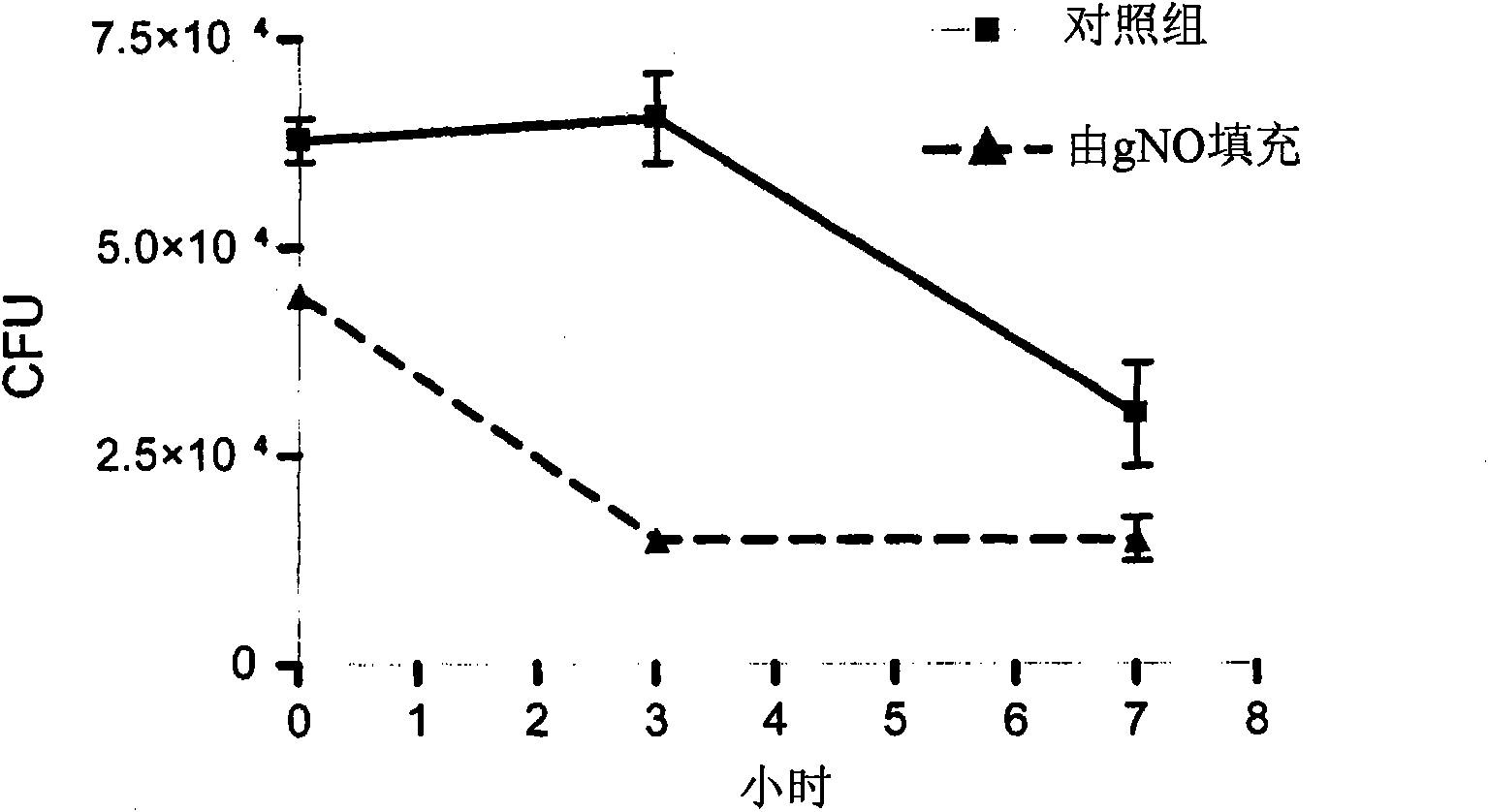
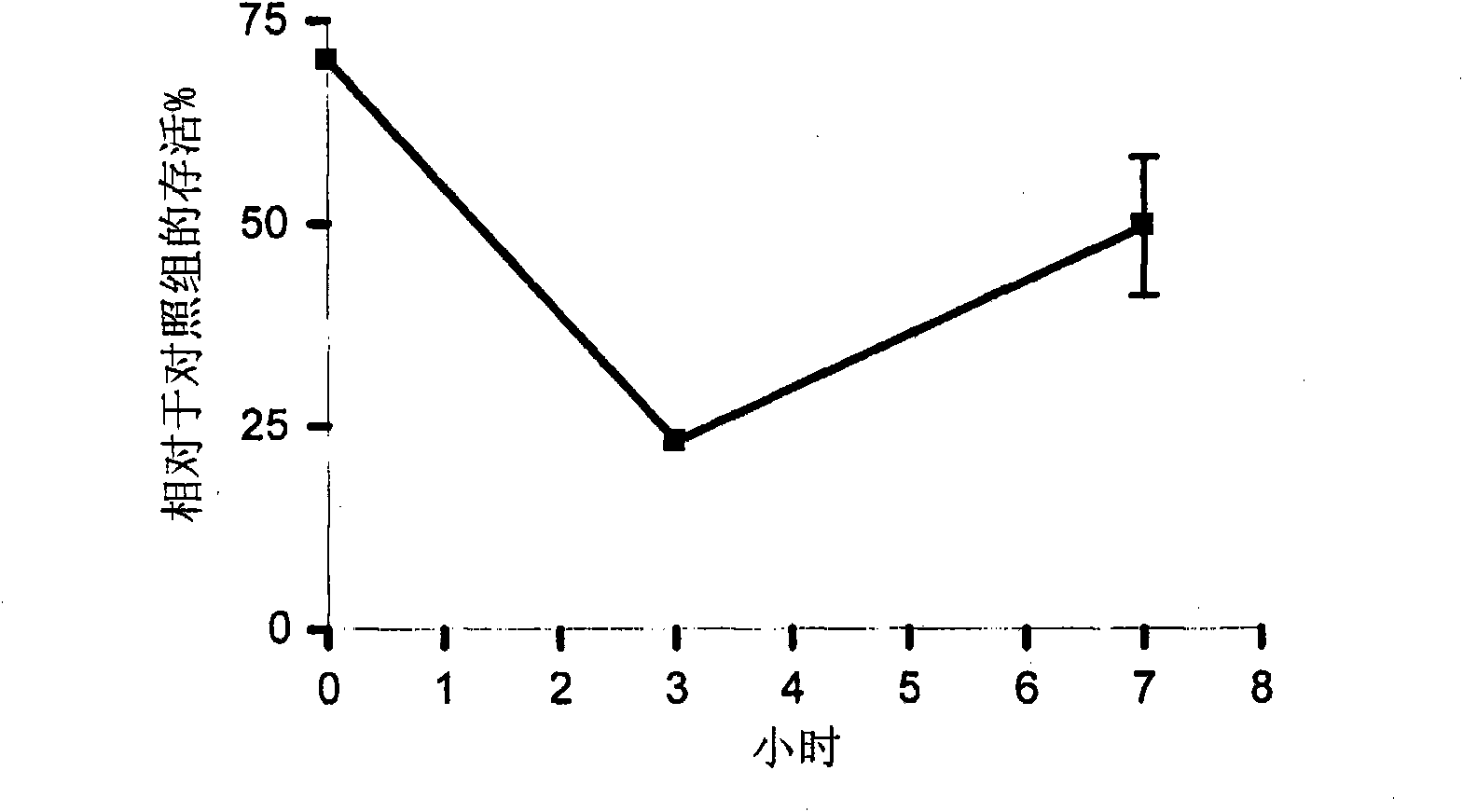
[0041] A plurality of vent tubes (1.25 mm Sheehy ring knobs, Cat. No. 23-40300; Inovotec International Inc., Jacksonville, Jacksonville, Florida, USA) were attached to a polytetrafluoroethylene (PTFE) substrate. , FL, USA)) into ventilated Petri dishes. Appropriate conduits were used to connect the Petri dishes to a manifold connected to a gas cylinder containing gNO manufactured by Airgas (Chicago Il, USA). The manifold was provided with an airflow controller adjusted to flow at 30cc min -1 A flow rate of 22,000 ppm gNO was delivered to each Petri dish for 22 hours. After completion of the gNO filling process, store the gNO filled PTFE vent tubes in an airtight container. After completing the gNO filling process, perform all handling of the vent tube using aseptic technique.
[0042] Stock cultures of Streptococcus pyogenes (ATCC #51878), Streptococcus pneumoniae (ATCC #10015), Moraxella catarrhalis (ATCC #25240) and Haemophilus influenzae (ATCC #35540) were maintained on ...
example 2
[0045]A plurality of vent tubes comprising a PTFE substrate (Armstrong Beveled venttube, circular 1.14 mm diameter fluoroplastic, from Gyrus ACMI, Cat. No. 140242; Florida, USA Innovite International, Jacksonville) in vented petri dishes. A suitable conduit was used to connect the petri dish to a manifold connected to a gas cylinder containing gNO manufactured by Elgers (Chicago, IL, USA). The manifold was provided with an airflow controller adjusted to flow at 30cc min -1 A flow rate of 22,000 ppm gNO was delivered to each Petri dish for 22 hours. After completion of the gNO filling process, store the gNO filled PTFE vent tubes in an airtight container. After completing the gNO filling process, perform all handling of the vent tube using aseptic technique.
[0046] A stock culture of methicillin-resistant Staphylococcus aureus (MRSA; ATCC #700698) was maintained on nutrient agar. MSRA is a strain of S. aureus known to be resistant to a variety of broad-spectrum antibiotic...
example 3
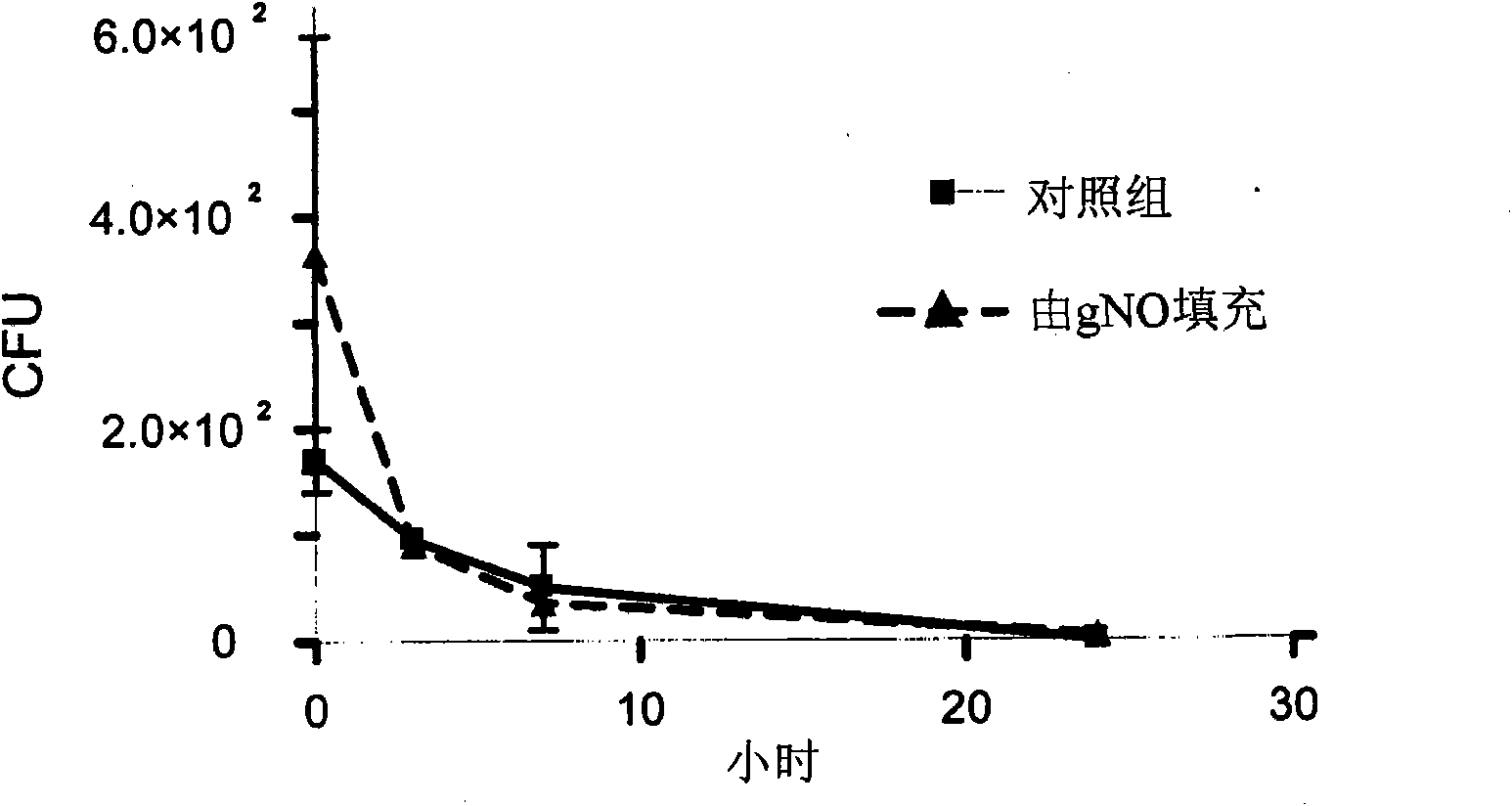
[0049] The first batch of vent tubes comprising a silicon substrate (T-Tubes, Silicone Myringotomy Tubing, 23-50600; Innovite International, Jacksonville, FL, USA) and the second batch of vent tubes comprising PTFE Ventilation tubes for the substrate (Hey ring knob, fluoroplastic myringotomy tubing, 23-40300; Innovit International, Jacksonville, FL, USA) were placed in vented petri dishes. A suitable conduit was used to connect the petri dish to a manifold connected to a gas cylinder containing gNO manufactured by Elgers (Chicago, IL, USA). The manifold was provided with an airflow controller adjusted to flow at 30cc min -1 A flow rate of 22,000 ppm gNO was delivered to each Petri dish for 22 hours. After completion of the gNO filling process, the silicon and PTFE vents filled with gNO were stored in airtight containers. After completing the gNO filling process, perform all handling of the vent tube using aseptic technique.
[0050] A stock culture of Staphylococcus aureus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com