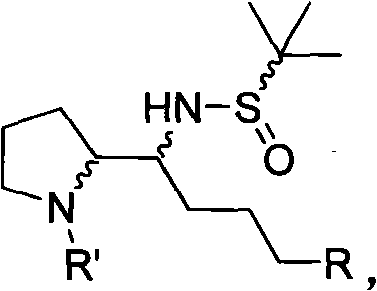
A type of pyrrolidine derivative, synthesis method and purposes thereof in synthesizing 2,2'-pyrrolidine
A synthesis method and technology of pyrrolidine are applied in directions such as organic chemistry to achieve the effects of wide sources, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
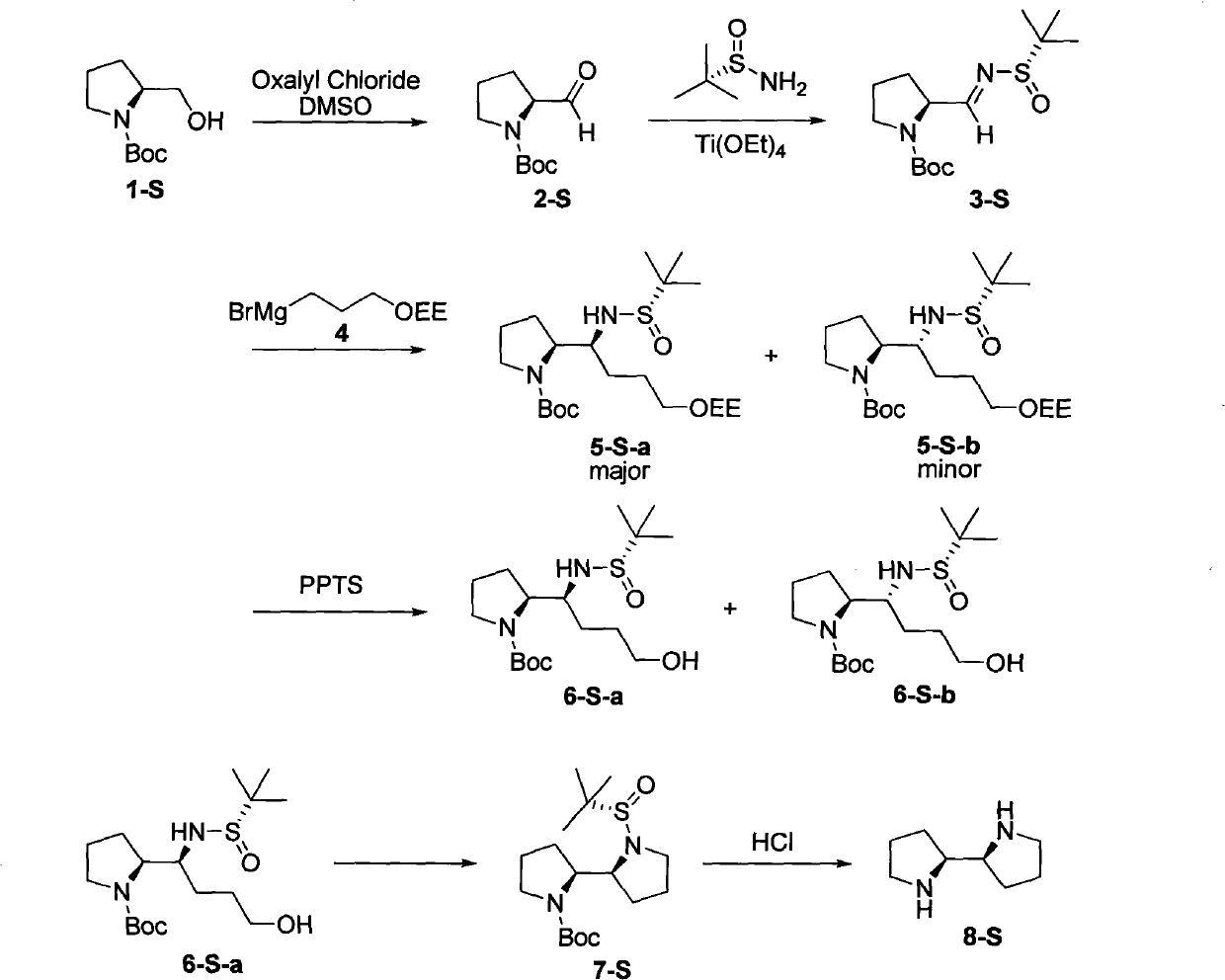
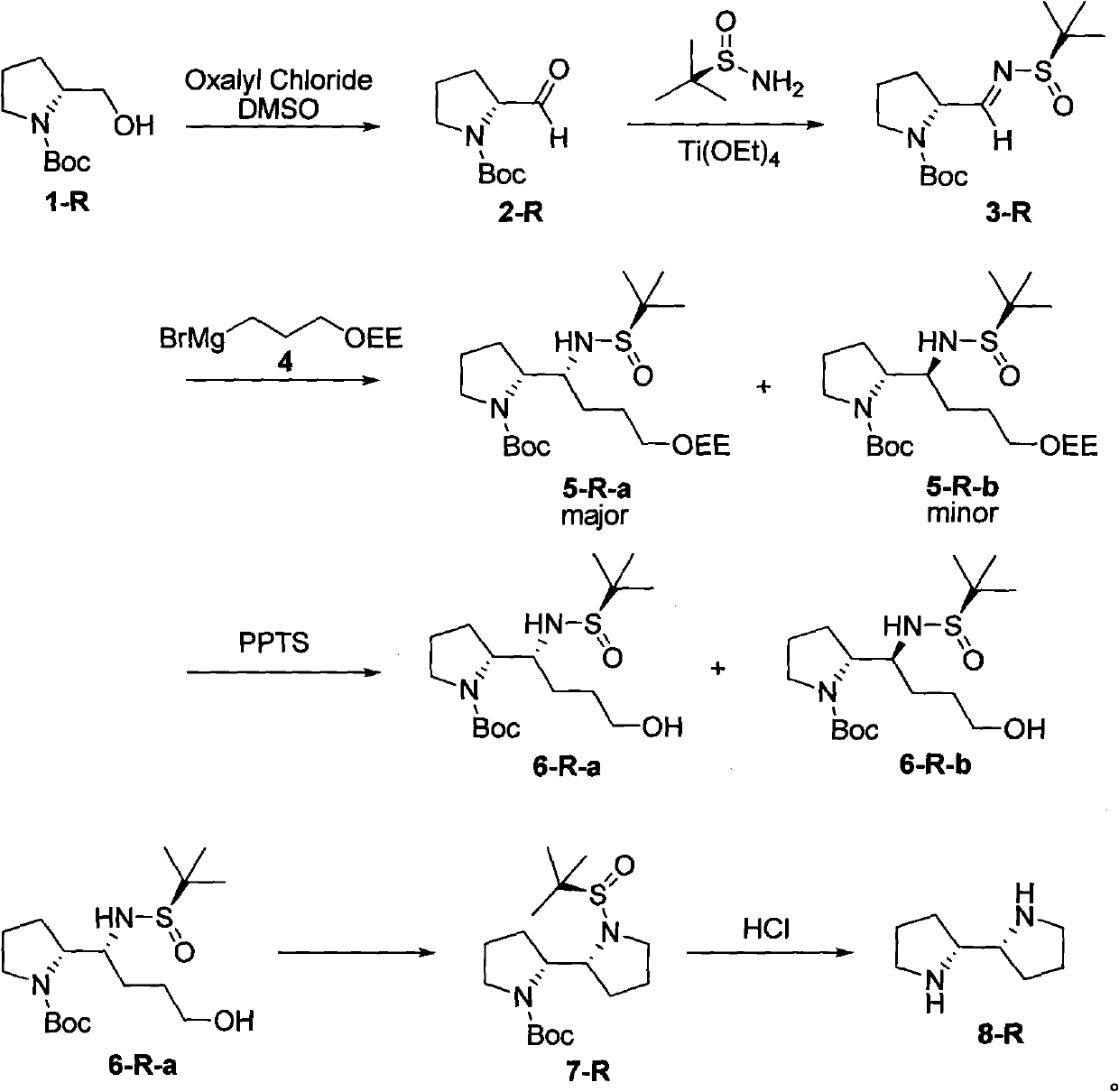
[0029] The synthesis of embodiment 1 compound (2-S)
[0030]
[0031] Dissolve 2.6ml oxalyl chloride (30mmol) in 60ml CH 2 Cl 2 , N 2 Under protection, the temperature dropped to -78°C. Add 4.6ml DMSO (65mmol) dropwise to the system at -78°C and stir for 30min. 5.01g compound (1-S) (25mmol) was dissolved in 40ml CH 2 Cl 2 , was slowly added dropwise to the reaction system, maintained at -78°C, and stirred for 1 h. Add 17.2ml NEt to the system 3 , After stirring at -78°C for 20 min, it was raised to room temperature. Add water to the system, separate the liquid, and the water phase is separated by CH 2 Cl 2 extraction. The organic phase was sequentially washed with saturated NH 4 Cl solution, saturated Na 2 CO 3 solution, washed with saturated brine, MgSO 4 After drying, the solvent was removed by rotary evaporation, and separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain 4.74 g (94%) of compound (2-S). [α] D 23 -90.1 (c1.1, C...
Embodiment 2
[0032] The synthesis of embodiment 2 compound (3-S)
[0033]
[0034] N 2 Under protection, 65.4g (328mmol) of compound (2-S) and 44.04g (S)-tert-butylsulfinamide (363mmol) were added to an eggplant-shaped bottle and dissolved in 370ml THF. Cool the system to 0°C, add Ti(OEt) 4 (331.6g, 1.45mol) in THF (450ml). The system was returned to room temperature and stirred overnight. Under ice bath, add saturated saline to the system to destroy excess Ti(OEt) 4 , and the resulting mixture was filtered through celite, which was washed with ethyl acetate. Separate the liquid, extract the inorganic phase with ethyl acetate, combine the organic phase, MgSO 4After drying, separation and purification by column chromatography (petroleum ether: ethyl acetate = 4:1), compound (3-S) (69 g, 69%) was obtained. [α] D 30 79.2 (c1.64, CHCl 3 ).IR (neat): 2978, 2932, 1699, 1626, 1393cm -1 . 1 H NMR (300MHz, CDCl 3 ): δ1.19(s, 9H), 1.41(s, 9H), 1.69-2.26(m, 4H), 3.32-3.58(m, 2H), 4.49...
Embodiment 3
[0035] The synthesis of embodiment 3 compound (6-S-a) and (6-S-b)
[0036]
[0037] Add 6.01g of magnesium chips (0.24mol) and 60ml of THF in a 250ml three-necked flask. 36ml Br(CH 2 ) 3 OEE (0.24mol) was added dropwise to the reaction system, and 60ml THF was gradually added during the dropwise addition, and the temperature of the system was maintained at 25-30°C. After the dropwise addition was completed, stir at room temperature for 1 h.
[0038] The Grignard reagent was calibrated by salicylaldehyde phenylhydrazone, and the concentration of the Grignard reagent was 0.75M.
[0039] 142ml of Grignard reagent (106.5mmol) was transferred to an eggplant flask and cooled to -48°C. CH of compound (3-S) (17.1 g, 56.5 mmol) 2 Cl 2 (100ml) solution was slowly added to the system at this temperature, and kept stirring at this temperature for 16h. The reaction was brought back to room temperature, stirred for 1 h, and saturated NH 4 The reaction was quenched with Cl solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com