Process for the preparation of alkyl 3,3-dialkoxypropionates
A dialkoxy propionate, process technology, applied in the field of continuous process, can solve problems such as difficult to handle ketene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of methyl 3,3-dimethoxypropionate
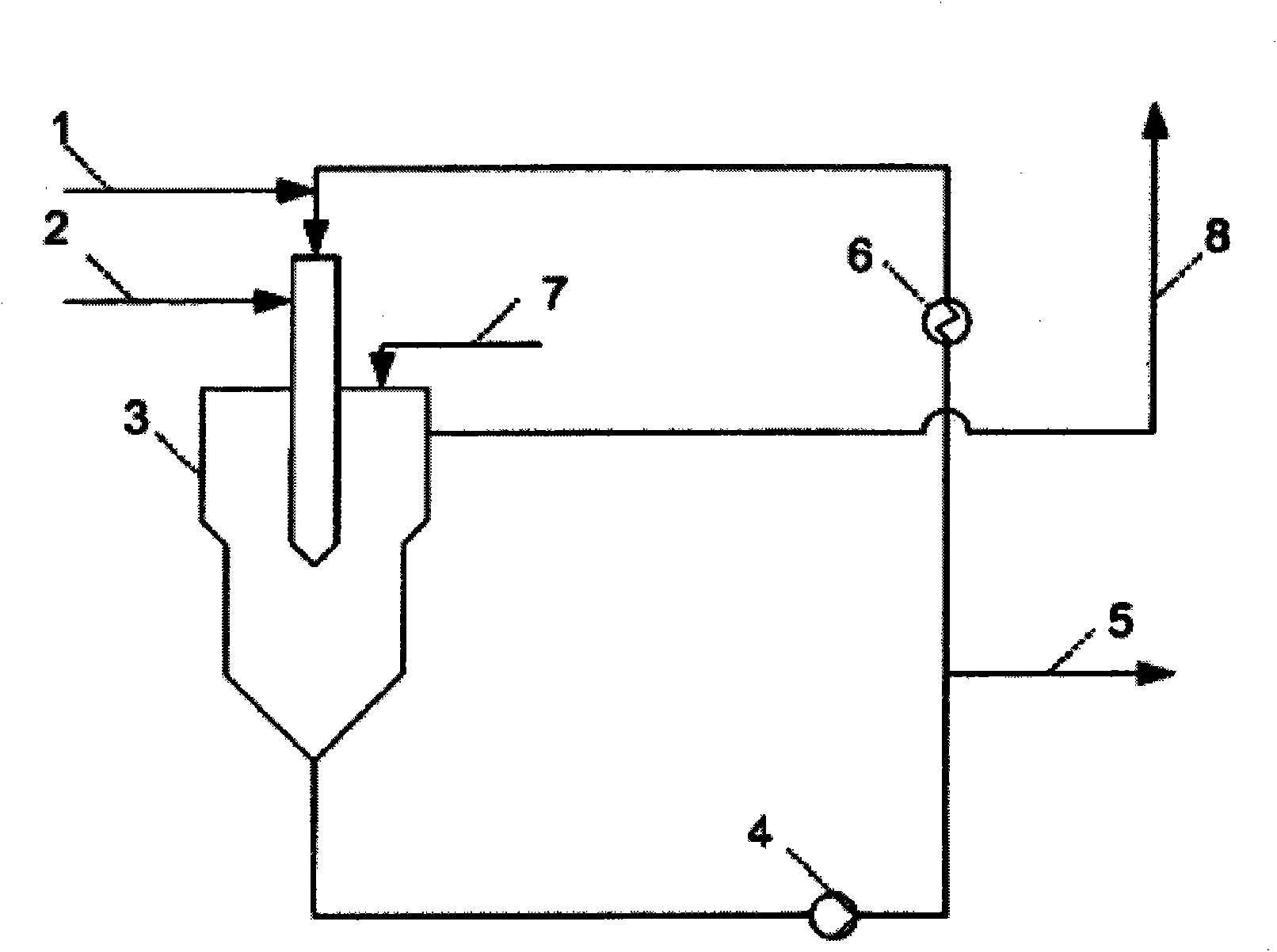
[0047] 150 kg / h (1.413 kmol / h) of trimethylorthoformate (Fluka) containing 1.5% by weight of montmorillonite K10 (Süd-Chemie) and 84 kg / h of ketene (ketene content about 70% , the rest is an inert gas, such as N 2 , CO and CO 2 , that is, net ketene about 59kg / h, equivalent to about 1.4kmol / h) simultaneously but separately into 620L injection reactor (see figure 1 ), which was inertized and cooled to an internal temperature of 0°C. Under a nitrogen atmosphere, the reaction mixture was maintained at a temperature of about 0°C and circulated in a loop by a circulation pump. The corresponding portion of the reaction mixture corresponding to the amount of starting material added is continuously passed into the holding tank. After filtration, the purity of the filtrate was determined by GC to be 80% methyl 3,3-dimethoxypropionate, 8% unreacted trimethyl orthoformate, 4% methyl 3-methoxypropionate -2-enoat...
Embodiment 2
[0050] Embodiment 2: the preparation of methyl 3-methoxyprop-2-enoate
[0051] Under a nitrogen atmosphere, 0.2 g (2 mmol) of methanesulfonic acid (Fluka) was added to 150 g of filtrate obtained in a similar manner as described in Example 1 in a distillation apparatus with a round bottom flask (about 85%, 0.86 mol of 3,3-dimethoxypropionate). Under a constant flow of nitrogen, the mixture was heated slowly to 160° C. and the methanol formed was distilled off directly. After 6 hours, the heating was turned off. The methyl 3-methoxy-prop-2-enoate obtained in this way is 88% pure (GC) and can be purified by rectification at 10 kPa. The yield of methyl 3-methoxyprop-2-enoate was 85 g (85%) (K 10kPa =95°C) with a purity of 99% (GC).
Embodiment 3
[0052] Embodiment 3: the preparation of methyl 3-methoxyprop-2-enoate
[0053] The reaction adopts 5 g (content 99%, 34 mmol) of purified distilled methyl 3,3-dimethoxypropionate and 25 mg (0.13 mmol) of p-toluenesulfonic acid monohydrate (Fluka) through a method similar to that of Example 2. way. The crude product obtained contained 91% (GC) of methyl 3-methoxyprop-2-enoate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com