Salt-free preparation method for substituted taurine
A technology of taurine and thiocarboxylic acid is applied in the field of salt-free preparation of taurine, and can solve problems such as troublesome desalination and purification processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
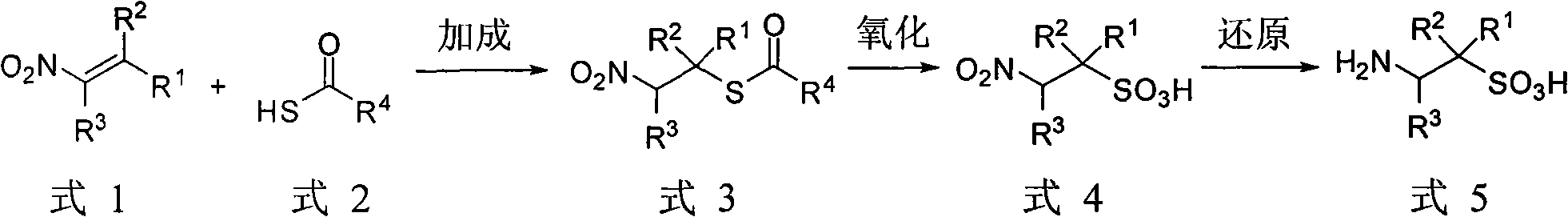
[0046] Preparation of 1-phenyl-2-aminoethanesulfonic acid (5a)
[0047] Dissolve 1-phenyl-2-nitroethylene (2.98g, 20mmol) in 20mL ether (or benzene, or THF), then add thioacetic acid (1.52g, 20mmol), and 200mg tributylamine, at room temperature Stir for 1h. After the reaction was completed, 20 mL of ether was added, followed by washing with 0.1 mol / L hydrochloric acid and deionized water respectively. The organic phase was dried, and the solvent was spun off. After recrystallization or column chromatography, 4.37 g of 1-phenyl-2-nitroethanethiol acetate was obtained, with a yield of 97%. It is a colorless crystal with a melting point of 126°C. 1 HNMR (300MHz, CDCl 3 )δ: 7.34~7.29(m, 5H, ArH), 5.29(t, J=7.5Hz, 1H, CHS), 4.84(d, J=7.5Hz, 2H, CHS 2 N), 2.36(s, 3H, CH3). 13 C NMR (75.5MHz, CDCl 3 )δ: 193.3, 135.6, 129.2, 128.8, 127.7, 77.9, 44.4, 30.3.
[0048] 30% LH 2 o 2 (10m) and 25mL of anhydrous formic acid were mixed and stirred in an ice-water bath for 0.5h. Then...
Embodiment 2
[0050] Preparation of 1-(4-chlorophenyl)-2-aminoethyl-1-sulfonic acid (5b)
[0051] According to the method described in Example 1, 1-(4-chlorophenyl)-2-aminoethyl-1-sulfonic acid was obtained from 1-phenyl-2-nitroethylene as a raw material, colorless crystals, melting point 342 ℃, yield 67%. 1 H NMR (D 2 O, 300MHz) (δ, ppm) 3.47 (dd, J=8.7, 13.4Hz, 1H in CH 2 N), 3.76(dd, J=6.9, 13.4Hz, 1H in CH 2 N), 4.27 (dd, J=6.9, 8.4Hz, 1H, CHS), 7.38 (m, 4H, ArH). 13 C NMR (D 2 O, 75.5MHz) (δ, ppm) 41.1, 61.9, 129.2, 129.4, 130.9, 134.9.
Embodiment 3
[0053] Preparation of 3-methyl-1-aminobutane-2-sulfonic acid (5c)
[0054] According to the method described in Example 1, 3-methyl-1-aminobutane-2-sulfonic acid was obtained from 1-nitro-3-methyl-1-butene as a raw material, colorless crystals, melting point 338- 340°C, yield 64%. 1 H NMR (D 2 O, 300MHz) (δ, ppm) 0.89 (d, J=7.0Hz, 3H, CH 3 ), 0.97 (d, J=7.0Hz, 3H, CH 3 ), 2.22 (dqq, J=3.3, 7.0, 7.0Hz, 1H, CH), 2.87 (ddd, J=2.9, 3.3, 10.1Hz, 1H, CHS), 3.18 (dd, J=10.1, 13.8Hz, 1H in CH 2 N), 3.26 (dd, J=2.9, 13.8Hz, 1H in CH2N). 13 C NMR (D 2 O, 75.5MHz) (δ, ppm) 17.1, 21.1, 28.0, 37.4, 62.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com