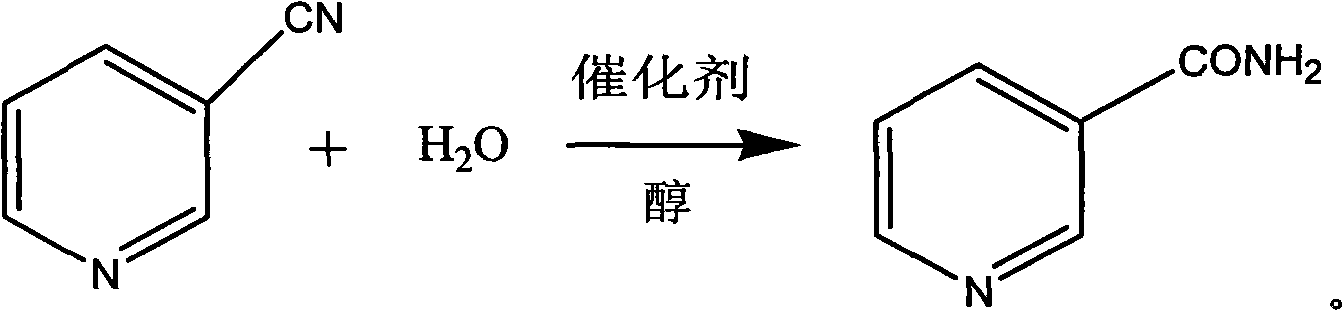
Method for preparing nicotinamide
A technology of nicotinamide and cyanopyridine, which is applied in the field of nicotinamide preparation, can solve problems such as the low pH value of nicotinamide products, difficulty in meeting requirements, and increased production costs, and achieve easy product separation, good product quality, and high production capacity. sufficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 nicotinamide
[0027] In a 1000ml four-necked flask, throw 20g (0.23mol) of manganese dioxide, 100g (0.96mol) of solid 3-cyanopyridine, and 400g of ethanol (wherein ethanol 380g, 8.25mol; water 20g) with a mass percentage concentration of 95%. , 1.11mol). Stir and heat up to 90°C to start heat preservation, keep the temperature at 90°C + 5°C for 6 hours and then end the reaction. After the reaction, the reaction product is sampled for detection by HPLC. Test results (HPLC): Based on the integral of the peak area normalization method, the mass percentage of nicotinamide was 99.7%, the mass percentage of nicotinic acid was 0.3%, and 3-cyanopyridine was not detected; the reaction conversion rate was 100%, Selectivity 99.7%. The reaction product was rotary evaporated to dryness, then taken out, and vacuum-dried at 60° C. for 10 hours to obtain 116.7 g of nicotinamide, the molar yield of nicotinamide was 99.49%, and the pH value was 7.0.
Embodiment 2
[0028] The preparation of embodiment 2 nicotinamide
[0029] In a 1000ml four-neck flask, put 20g (0.23mol) of manganese dioxide, 100g (0.96mol) of solid 3-cyanopyridine, 450g (6.07mol) of n-butanol and 22g (1.22mol) of water. Stir and heat up to 80°C and start to keep warm, keep the temperature at 80°C+7°C for 8 hours and then end the reaction. After the reaction, the reaction product is sampled and detected by HPLC. Test results (HPLC): Based on the integral of the peak area normalization method, the mass percentage of nicotinamide was 99.5%, the mass percentage of nicotinic acid was 0.5%, and 3-cyanopyridine was not detected; the reaction conversion rate was 100%, Selectivity 99.5%. The reaction product was rotary evaporated to dryness and then taken out. After vacuum drying at 80° C. for 16 hours, 116.9 g of nicotinamide was obtained. The molar yield of nicotinamide was 99.66%, and the pH value of nicotinamide was 6.9.
Embodiment 3
[0030] The preparation of embodiment 3 nicotinamide
[0031] In a 1000ml four-necked flask, throw 15g (0.173mol) of manganese dioxide, 100g (0.96mol) of solid 3-cyanopyridine, 300g (9.36mol) of methanol, and 18g (1.00mol) of water. Stir and heat up to 95°C to start heat preservation, keep the temperature at 95°C ± 5°C for 7 hours and then end the reaction. After the reaction, the reaction product is sampled for detection by HPLC. Test results (HPLC): Based on the integral of the peak area normalization method, the mass percentage of nicotinamide was 99.4%, the mass percentage of nicotinic acid was 0.6%, and 3-cyanopyridine was not detected; the reaction conversion rate was 100%, Selectivity 99.4%. The reaction product was rotary evaporated to dryness, then taken out, and vacuum-dried at 70° C. for 8 hours to obtain 116.5 g of nicotinamide, the molar yield of nicotinamide was 99.32%, and the pH value was 6.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com