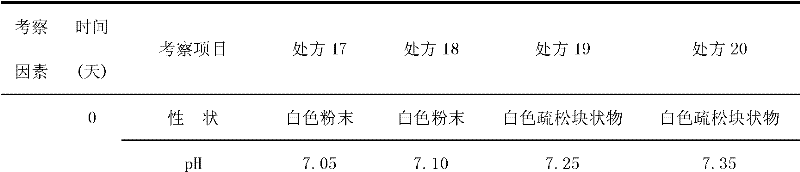
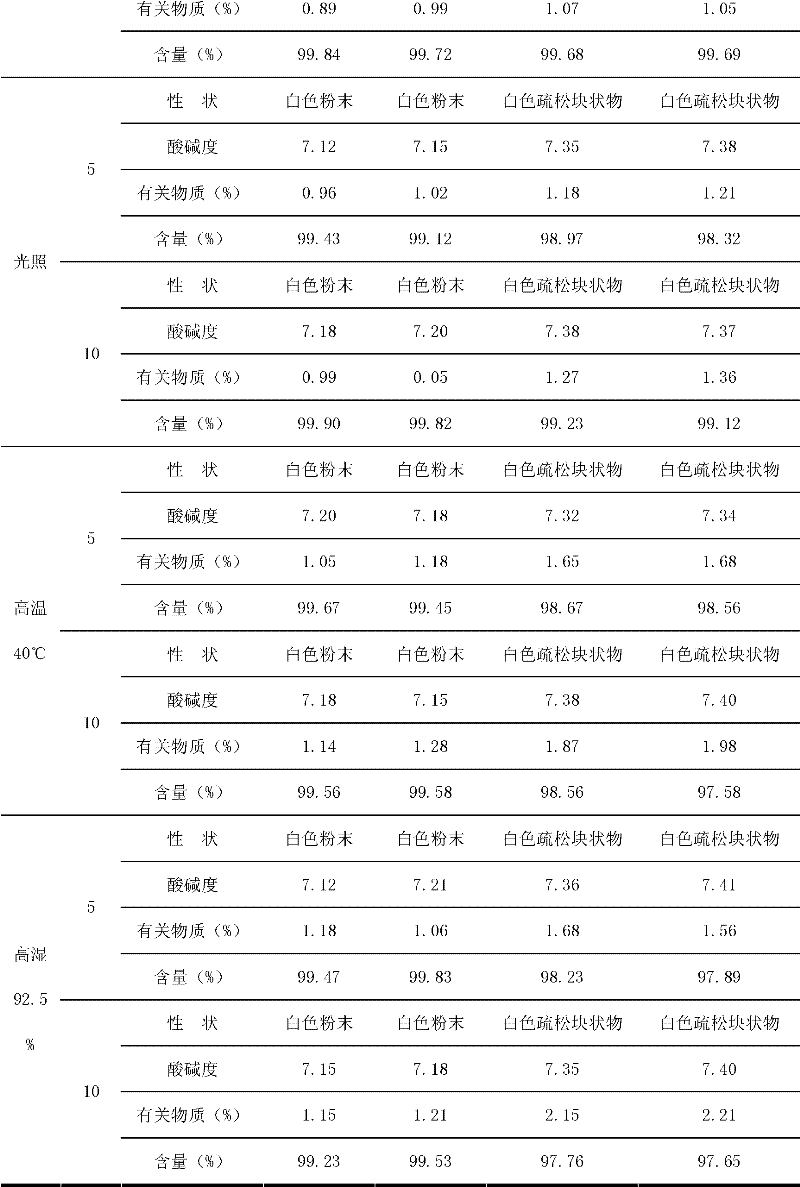
Asarin composite freeze-dried powder for injection
A technology of freeze-dried powder injection and asarone, which is applied in the direction of freeze-dried delivery, drug combination, powder delivery, etc., can solve the problems of large proportion of excipients added, many types of excipients, and unsatisfactory reconstitution effect, etc., to achieve guaranteed The effects of stability, improving solubility, and reducing the amount of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The composition is:
[0046] Asarum 8.0g
[0047] 95% ethanol 40ml
[0048] Mannitol 80.0g
[0049] Add water for injection to 1000ml
[0050] After lyophilization, 500 bottles are made.
[0051] (1) Prepare a 0.1% polysorbate-80 deionized water solution as an anti-solvent;
[0052] (2) Prepare an ether solution with a mass percentage of 0.1g / ml asarone as a solvent;
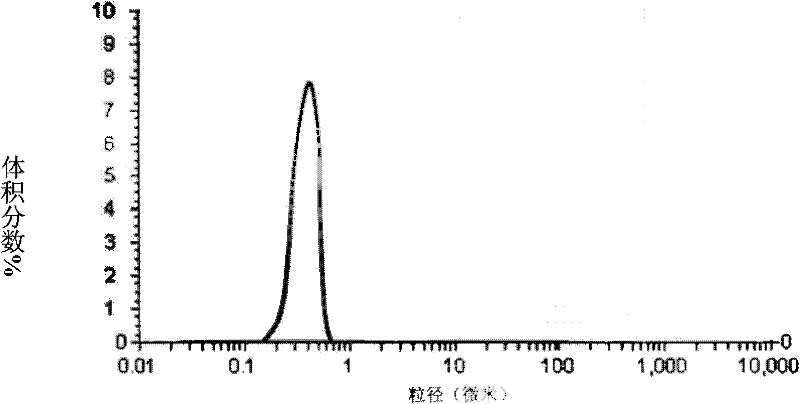
[0053] (3) Under the conditions of 2~6℃ and stirring speed of 600 rpm, add solvent to the anti-solvent quickly, and the volume ratio of solvent to anti-solvent is 1:15; after white crystals are produced, the crystals and solution are heated at 42℃ Aging for 2 hours under the conditions, filtering, washing, filter cake dispersion and spray drying; the obtained ultrafine particles of Asarone are obtained by laser particle size measuring instrument to obtain CSD curve and figure 1 the same;
[0054] (4) Dissolving the superfine particles of Asarone in 95% ethanol in proportion to obtain liquid A;
[0055] (5) Dissolve ma...
Embodiment 2
[0058] The prescription is:
[0059] Asarum 8.0g
[0060] 100% ethanol 80ml
[0061] Mannitol 80.0g
[0062] Add water for injection to 1000ml
[0063] After lyophilization, 500 bottles are made.
[0064] (1) Prepare a 0.5% polysorbate-80 deionized water solution as an anti-solvent;
[0065] (2) Prepare an ether solution with a mass percentage of 1g / ml Asarone as a solvent;
[0066] (3) Under the conditions of 6°C and stirring speed of 1200 rpm, the solvent was quickly added to the anti-solvent, and the volume ratio of the solvent to the anti-solvent was 1:21; after white crystals were produced, the crystals and the solution were kept at 55°C After aging for 5 hours, after filtering, washing, filter cake dispersion and spray drying, the obtained ultrafine particles of Asarone are obtained by laser particle size measuring instrument to obtain CSD curve and figure 1 the same;
[0067] (4) Dissolving the ultrafine particles of Asarone in 100% ethanol in proportion to obtain liquid A;
[0068]...
Embodiment 3
[0071] The prescription is:
[0072] Asarum 6.0g
[0073] 98% ethanol 40ml
[0074] Mannitol 24.0g
[0075] Add water for injection to 1000ml
[0076] After lyophilization, 500 bottles are made.
[0077] (1) Prepare a 0.25% polysorbate-80 mass percent deionized water solution as an anti-solvent;
[0078] (2) Prepare an ether solution with a mass percentage of 0.5g / ml asarone as a solvent;
[0079] (3) Under the conditions of 4℃ and stirring speed of 1000 rpm, add solvent to the anti-solvent quickly, and the volume ratio of solvent to anti-solvent is 1:20; after white crystals are produced, the crystals and solution are kept at 45℃ After aging for 3 hours, filtering, washing, filter cake dispersion and spray drying; the obtained ultrafine particles of Asarone were obtained by laser particle size measuring instrument to obtain the CSD curve and figure 1 the same;
[0080] (4) Dissolving the ultrafine particles of Asarone in 98% ethanol in proportion to obtain liquid A;
[0081] (5) Dissolve ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com