Cefmetazole sodium composition powder injection for injection
A technology of cefmetazole sodium and its composition, which is applied in the field of chemical pharmacy, can solve the problems of small particle size and uneven particle size distribution, and achieve the effect of qualified product quality and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] [Example 1] Preparation of Cefmetazole Sodium Crystals
[0061] a) At room temperature, 14kg of cefmetazole sodium was dissolved in 10L of water to obtain a 0.4kg / L aqueous solution of cefmetazole sodium;
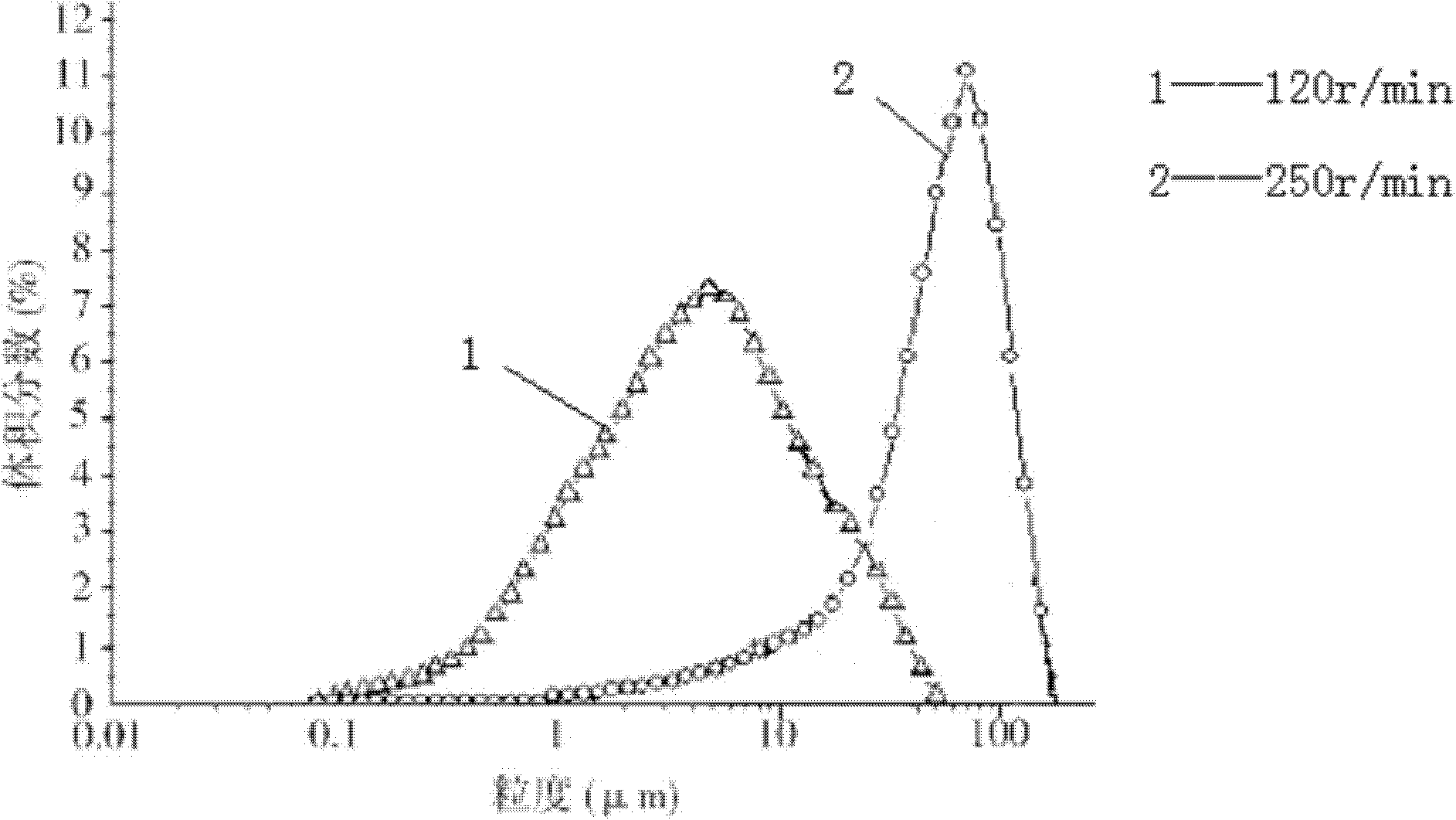
[0062] b) Add 70L of absolute ethanol at a rate of 10ml / min to the above-mentioned cefmetazole sodium aqueous solution under stirring at a stirring speed of 250r / min, and turbidity occurs;
[0063] c) Add a mixed solution of 70L acetone and isopropanol (the volume ratio of acetone and isopropanol is 1:2) to the above cloudy solution with a flow rate of 5ml / min under stirring at a stirring speed of 120r / min ), a large number of crystals are precipitated;
[0064] d) Cultivate crystals at a temperature of 22° C. for 20 minutes, filter, wash the filter cake three times with absolute ethanol, and dry in vacuum to obtain 13.3 kg of cefmetazole sodium crystals.
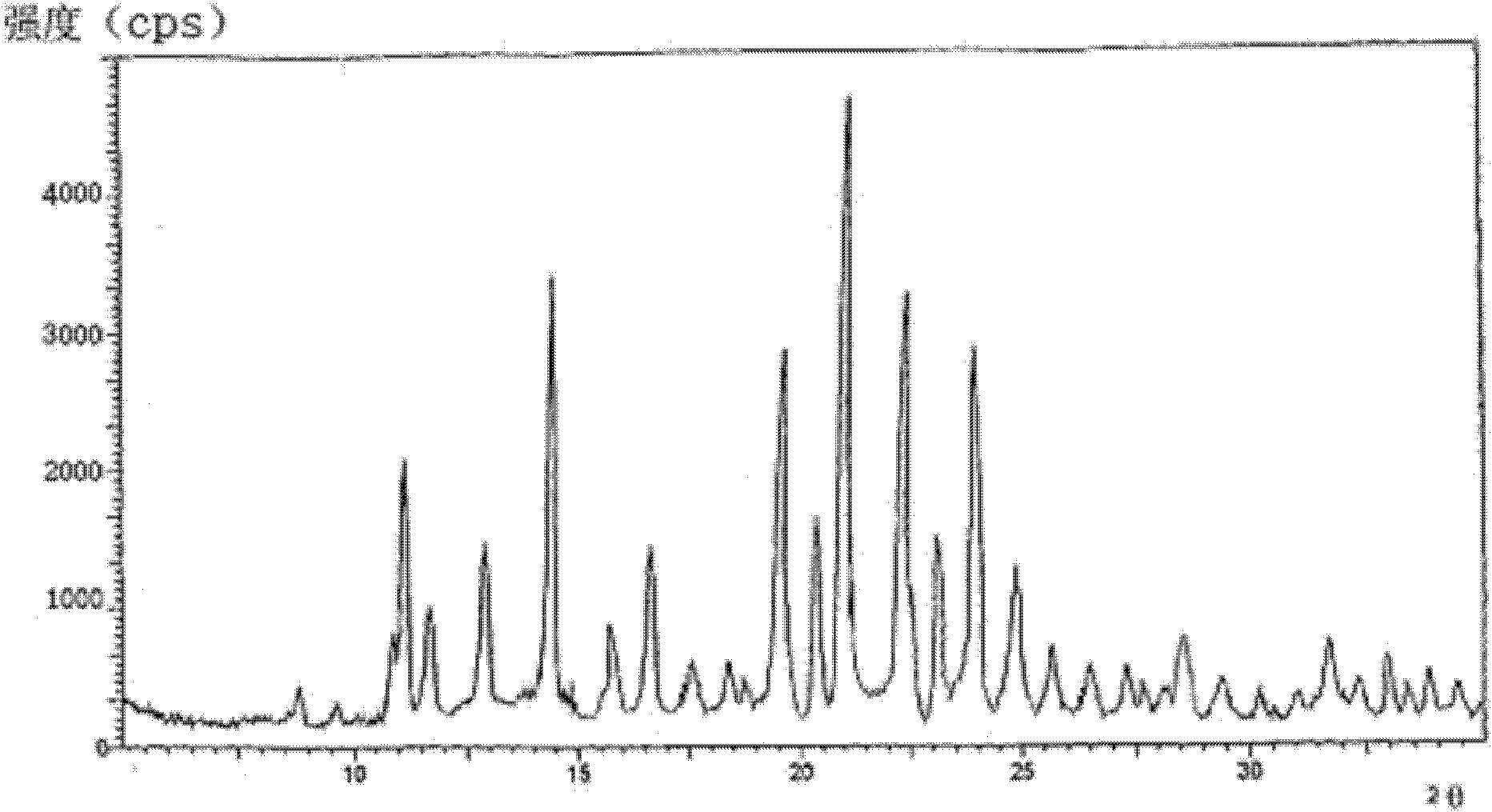
[0065] The main particle size of the obtained cefmetazole sodium crystal is 80 μm, and the characteristic pea...
Embodiment 2
[0066] [Example 2] Preparation of Cefmetazole Sodium Crystals
[0067] a) At room temperature, 16kg of cefmetazole sodium was dissolved in 10L of water to obtain a 0.6kg / L aqueous solution of cefmetazole sodium;
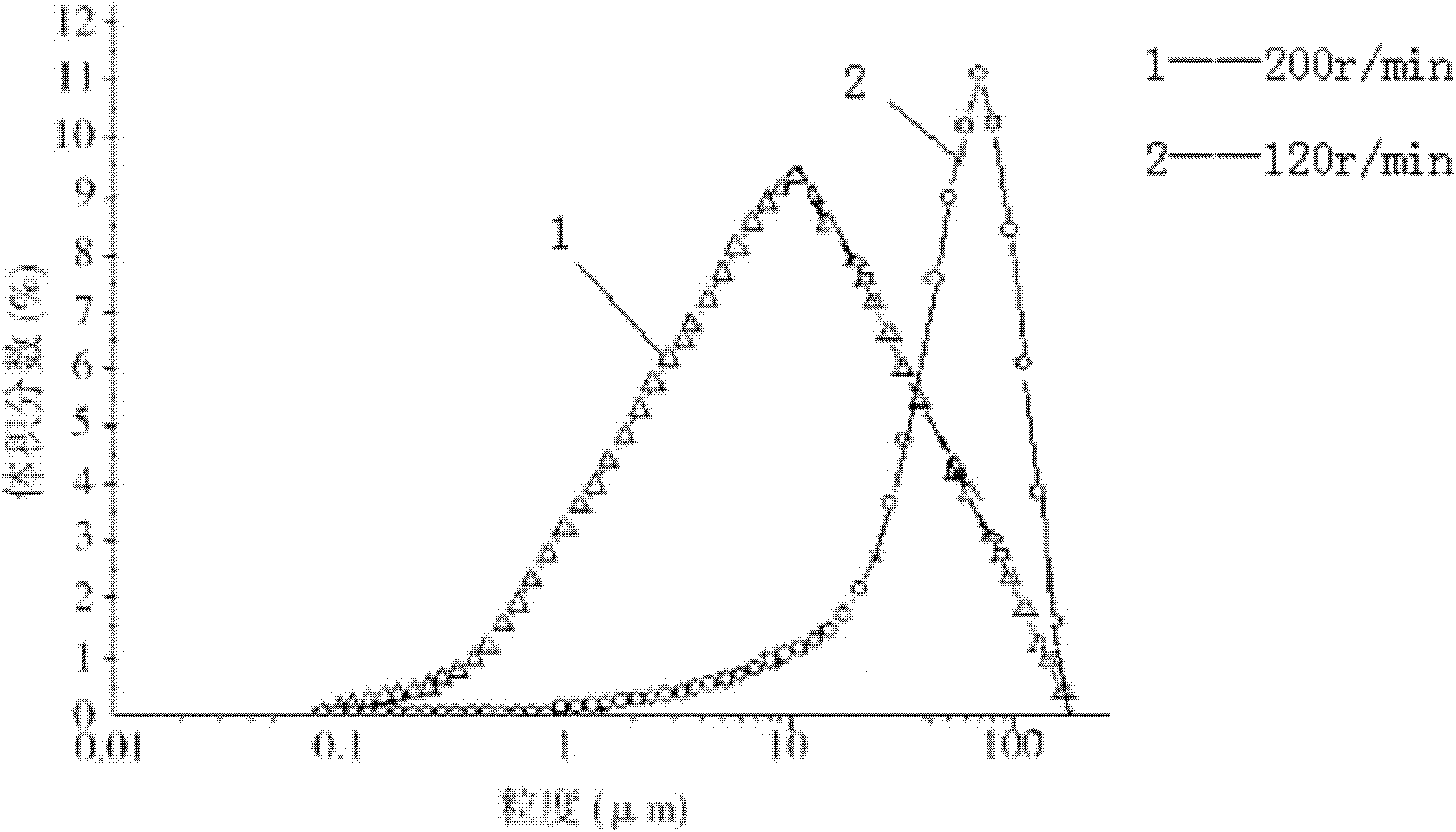
[0068] b) Add 75L of absolute ethanol at a rate of 15ml / min to the above-mentioned cefmetazole sodium aqueous solution under stirring at a stirring speed of 300r / min, and turbidity occurs;
[0069] c) Add the mixed solution of 150L acetone and isopropanol (the volume of acetone and isopropanol is 1:4) to the above-mentioned turbid solution under stirring at a stirring speed of 180r / min with a flow rate of 8ml / min , a large number of crystals precipitated;
[0070] d) Cultivate crystals at a temperature of 28° C. for 30 minutes, filter, wash the filter cake three times with absolute ethanol, and dry in vacuum to obtain 15.4 kg of cefmetazole sodium crystals.
[0071] The main particle size of the obtained cefmetazole sodium crystals was 120 μm, and the X-ray powder ...
Embodiment 3
[0072] [Example 3] Preparation of Cefmetazole Sodium Crystals
[0073] a) At room temperature, 15kg of cefmetazole sodium was dissolved in 10L of water to obtain a 0.5kg / L aqueous solution of cefmetazole sodium;
[0074] b) Add 73L of absolute ethanol at a rate of 12ml / min to the above aqueous solution of cefmetazole sodium under stirring at a stirring speed of 280r / min, and turbidity occurs;
[0075] c) Add a mixed solution of 219L acetone and isopropanol (the volume ratio of acetone and isopropanol is 1:3) to the above cloudy solution with a flow rate of 6ml / min under stirring at a stirring speed of 160r / min ), a large number of crystals are precipitated;
[0076] d) Cultivate crystals at a temperature of 25° C. for 25 minutes, filter, wash the filter cake three times with absolute ethanol, and dry in vacuum to obtain 14.4 kg of cefmetazole sodium crystals.
[0077] The main particle size of the obtained cefmetazole sodium crystals was 100 μm, and the X-ray powder diffract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Master granularity | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
| Master granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com