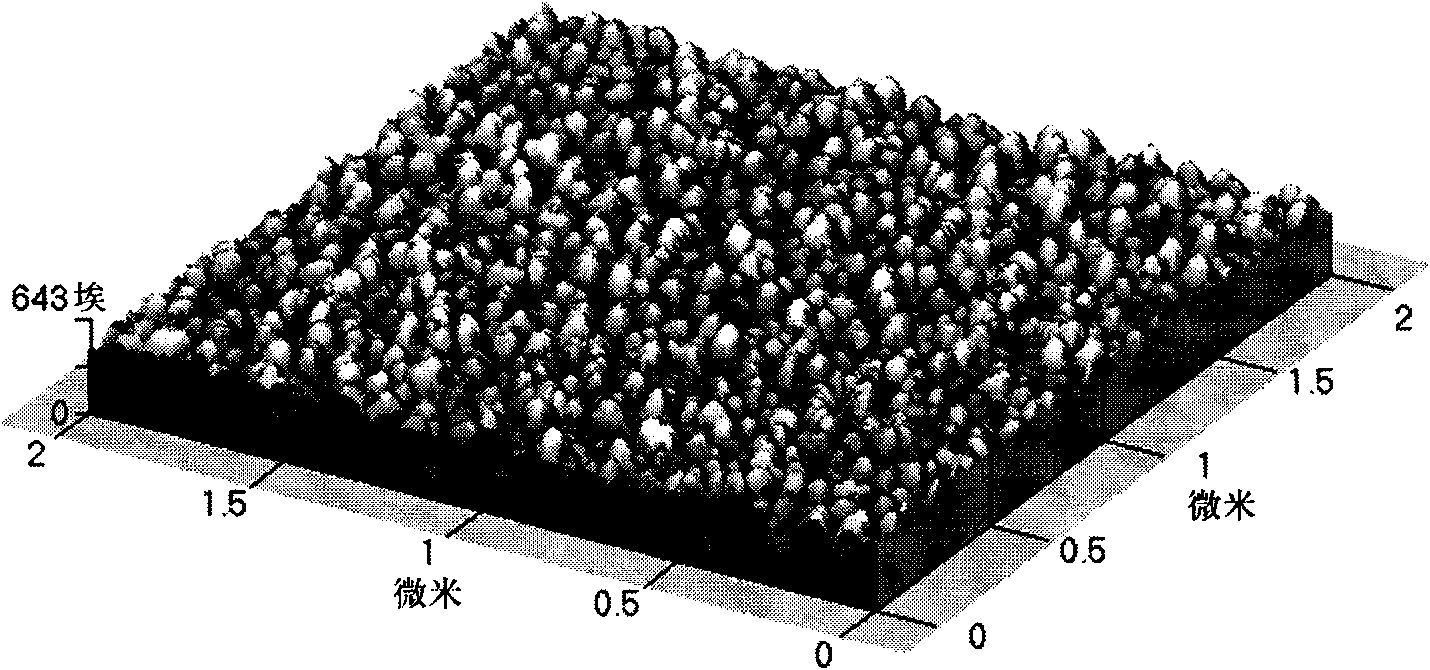
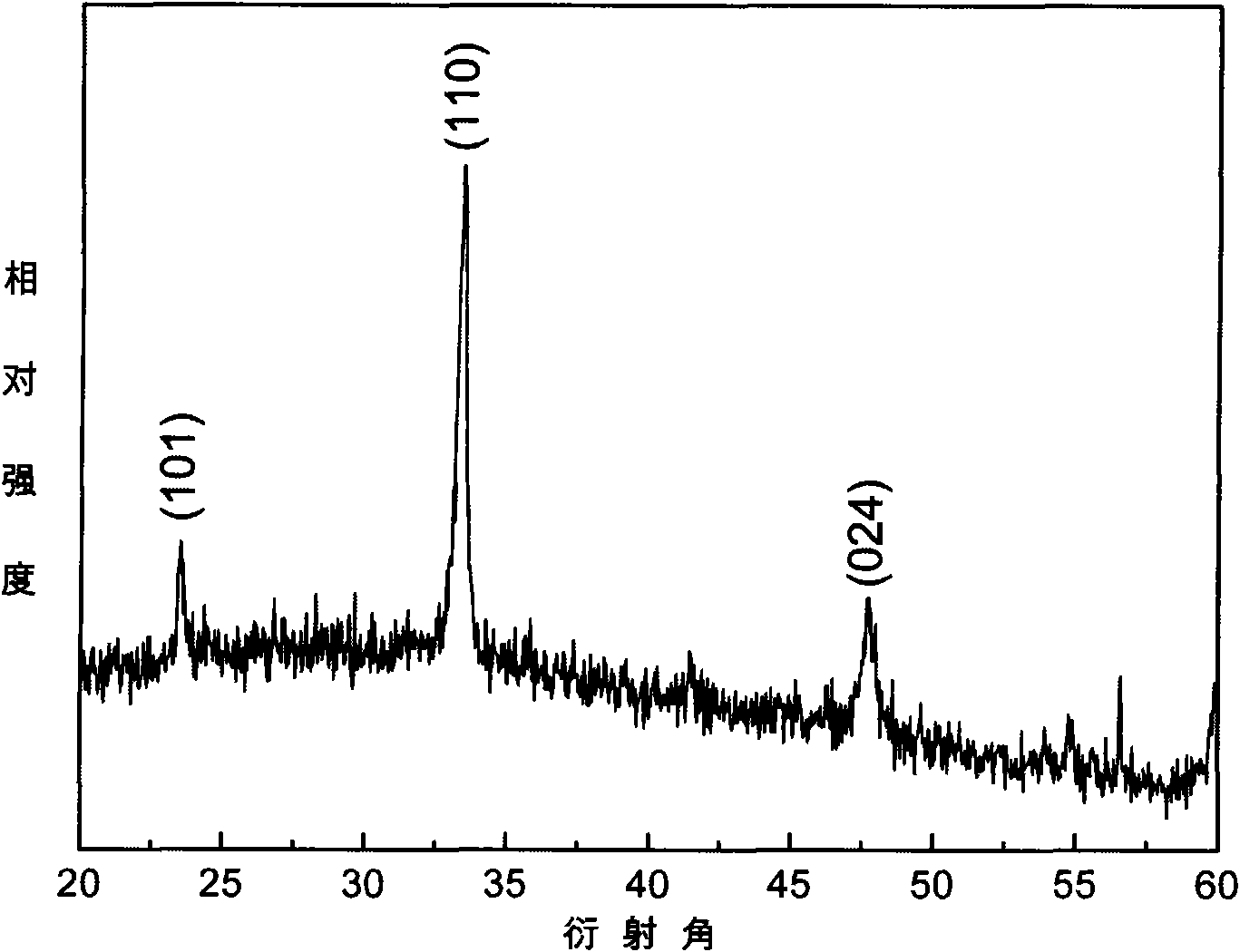
Method for preparing lanthanum nickel oxide thin-film material
A thin-film material, lanthanum nickelate technology, is applied in the field of preparation of lanthanum nickelate thin-film materials, can solve the problems of inaccurate stoichiometric ratio of precursor solution, difficulty in long-term storage, increased cost, etc. Low cost effect of process and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The specific steps of preparation are as follows: step 1, according to the molar ratio of nickel: lanthanum of 1:1, after weighing nickel acetate and lanthanum acetate, using propionic acid as solvent, first add nickel acetate to it; and place it in 40 Stir at ℃ for 30min, then add ethanolamine therein, place it at 40℃ and stir for 30min until the nickel acetate is completely dissolved to obtain a nickel acetate solution; wherein, the volume of propionic acid: the number of moles of nickel acetate: the volume of ethanolamine is 10L: 1 mole : 0.03L. Then, first add lanthanum acetate to the nickel acetate solution, place it at 40°C and stir for 30 minutes, then place it at room temperature and stir until it completely dissolves and becomes transparent to obtain a mixed solution; wherein, the moles of lanthanum in lanthanum acetate : The molar number of nickel in nickel acetate is 1:1. Afterwards, the mixed solution is filtered to obtain the lanthanum nickelate precursor ...
Embodiment 2
[0020] The specific steps of preparation are as follows: step 1, according to the molar ratio of nickel: lanthanum of 1:1, after weighing nickel acetate and lanthanum acetate, using propionic acid as solvent, first add nickel acetate to it, and place it at 48 Stir at ℃ for 25min, then add ethanolamine therein, place it at 48℃ and stir for 25min until the nickel acetate is completely dissolved to obtain a nickel acetate solution; wherein, the volume of propionic acid: the number of moles of nickel acetate: the volume of ethanolamine is 13L: 1 mole : 0.04L. Then, first add lanthanum acetate to the nickel acetate solution, place it at 48°C and stir for 25 minutes, then place it at room temperature and stir until it completely dissolves and becomes transparent to obtain a mixed solution; wherein, the molar number of lanthanum in lanthanum acetate : The molar number of nickel in nickel acetate is 1:1. Afterwards, the mixed solution is filtered to obtain the lanthanum nickelate pre...
Embodiment 3
[0022]The specific steps of preparation are: step 1, according to the molar ratio of nickel: lanthanum of 1:1, after weighing nickel acetate and lanthanum acetate, using propionic acid as solvent, first add nickel acetate to it, and place it at 55 Stir at ℃ for 20min, then add ethanolamine therein, place it at 55℃ and stir for 20min until nickel acetate is completely dissolved to obtain a nickel acetate solution; wherein, the volume of propionic acid: the number of moles of nickel acetate: the volume of ethanolamine is 15L: 1 mole : 0.05L. Then, first add lanthanum acetate to the nickel acetate solution, place it at 55°C and stir for 20 minutes, then place it at room temperature and stir until it completely dissolves and becomes transparent to obtain a mixed solution; wherein, the number of moles of lanthanum in lanthanum acetate : The molar number of nickel in nickel acetate is 1:1. Afterwards, the mixed solution is filtered to obtain the lanthanum nickelate precursor colloi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com