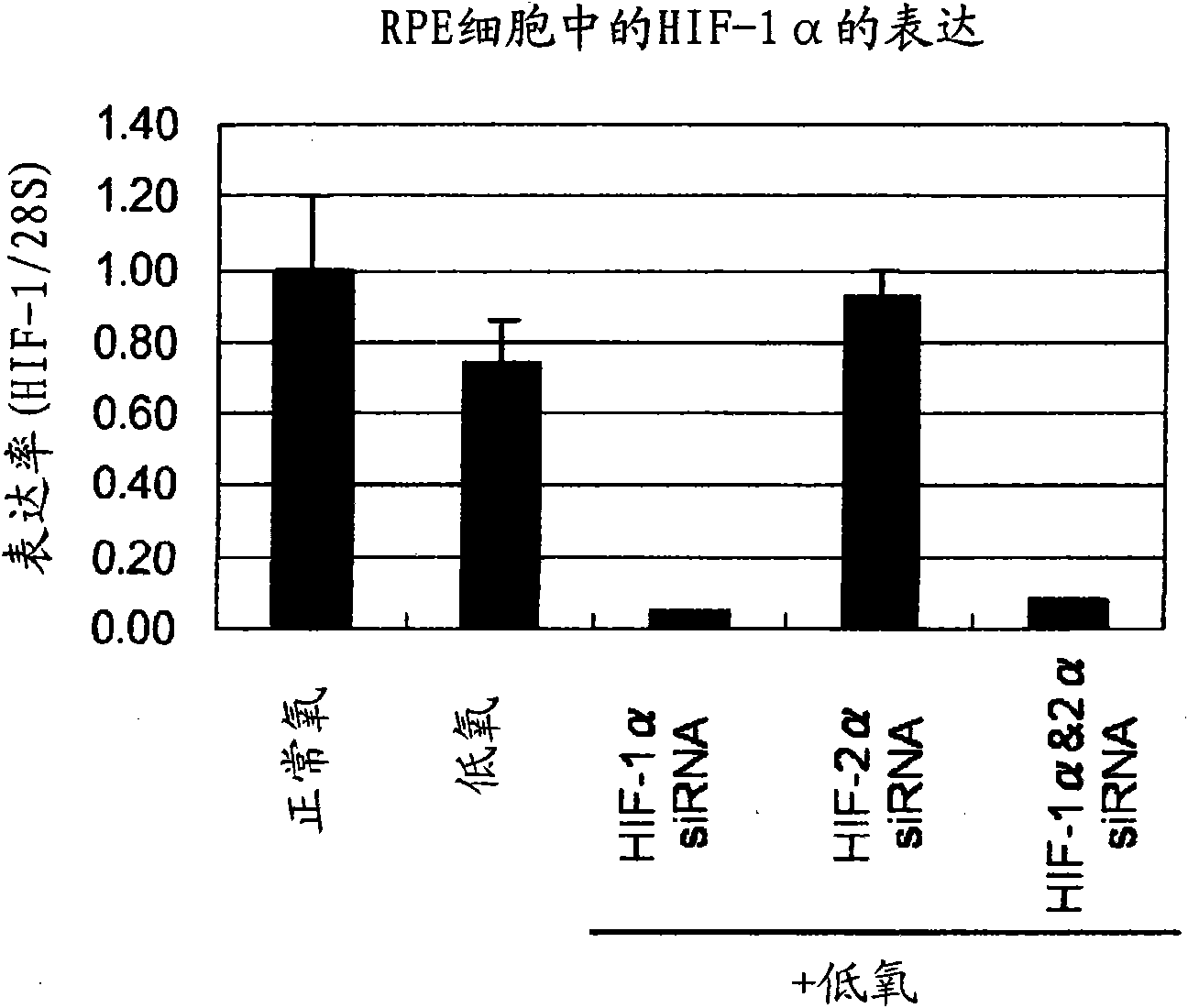
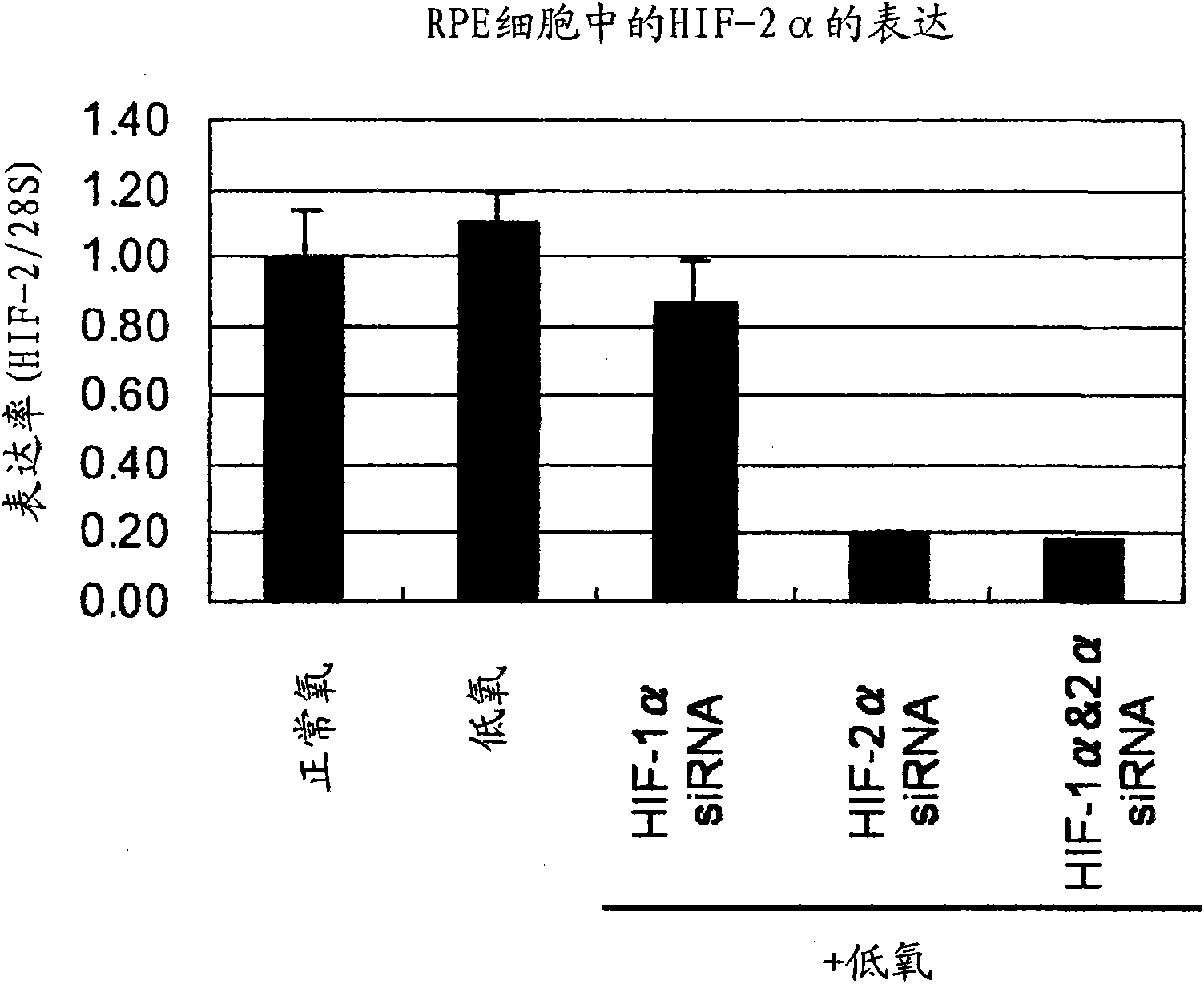
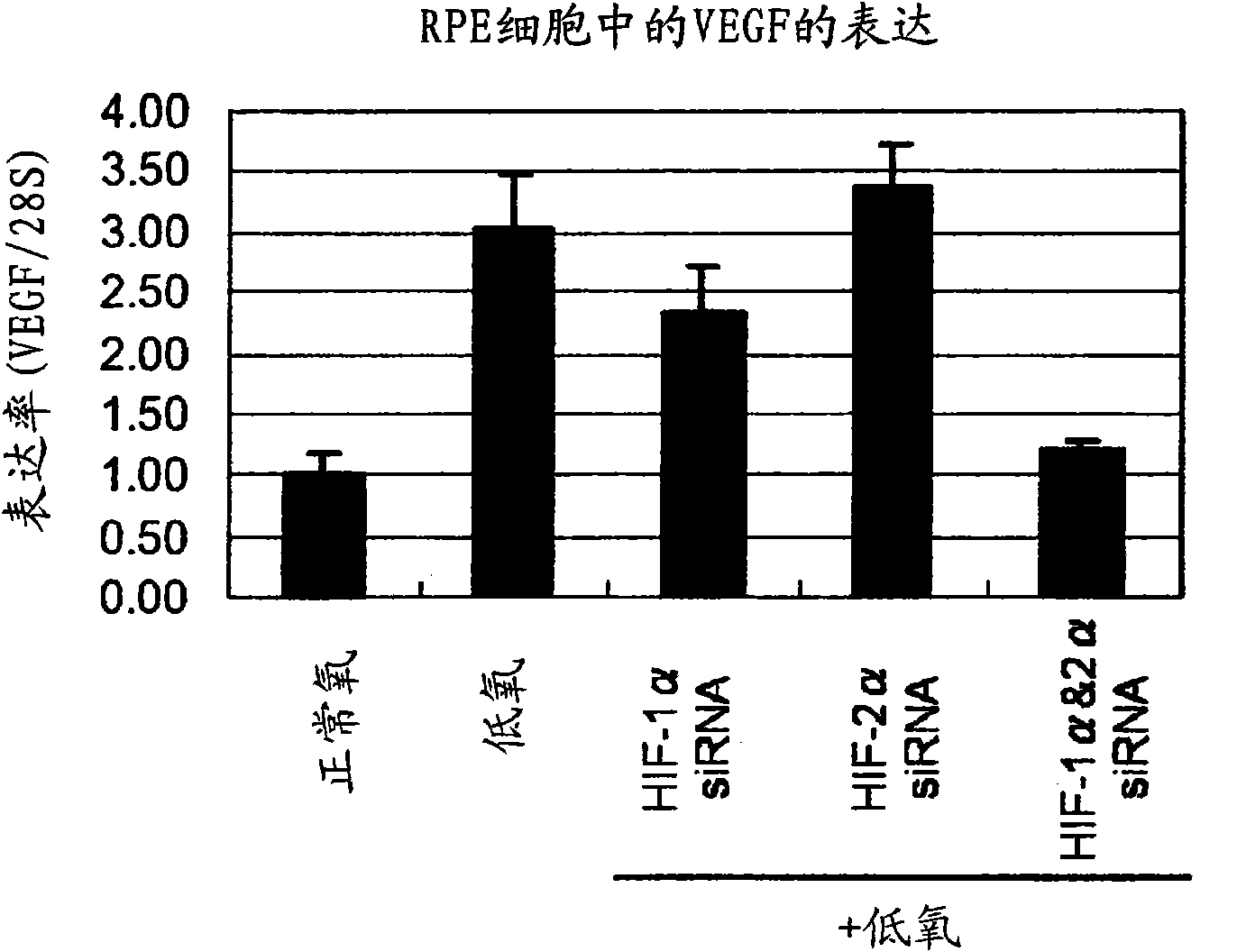
Pharmaceutical containing HIF-1alpha alpha and HIF-2alpha alpha expression inhibitor
A technology of HIF-1 and HIF-2, which can be used in medical preparations, drug combinations, genetic material components, etc. containing active ingredients, and can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0130] Examples are given below to further describe the present invention in detail, but the present invention is not limited thereto.
preparation example 1
[0131] Preparation example 1: Injection for intravitreal administration
[0132] Injections for intravitreal administration shown below were prepared according to conventional methods.
[0133] HIF-1α siRNA:
[0134] Sense-GGA ACC UGA UGC UUU AAC UTT (SEQ ID NO.5) 1mg
[0135] Antisense-AGU UAA AGC AUC AGG UUC CTT (SEQ ID NO.11) 1mg
[0136] HIF-2α siRNA:
[0137] Sense-CGG AGG UGU UCU AUG AGC UTT (SEQ ID NO.9) 1mg
[0138] Antisense-AGC UCA UAG AAC ACC UCC GTC (SEQ ID NO.15) 1mg
[0139] Sodium dihydrogen phosphate 0.1g
[0141] Appropriate amount of sodium hydroxide
[0142] Appropriate amount of sterile purified water
[0143]
[0144] Total 100mL
[0145] (pH7)
preparation example 2
[0146] Formulation example 2: Injection for intravitreal administration
[0147] Injections for intravitreal administration shown below were prepared according to conventional methods.
[0148] HIF-1α siRNA:
[0149] Sense-GGG UAA AGA ACA AAA CAC A TT (SEQ ID NO.6) 0.1mg
[0150] Antisense - UGU GUU UUG UUC UUU ACC C TT (SEQ ID NO.12) 0.1mg
[0151] HIF-2α siRNA:
[0152] Sense-GGU UUU GUU GCU AGC CCU U TT (SEQ ID NO.10) 0.1mg
[0153] Antisense-AAG GGC UAG CAA CAA AAC C TT (SEQ ID NO.16) 0.1mg
[0154] Sodium dihydrogen phosphate 0.1g
[0155] Glycerin 2.5g
[0156] Appropriate amount of sodium hydroxide
[0157] Appropriate amount of sterile purified water
[0158]
[0159] Total 100mL
[0160] (pH 7)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com