Polybrominated diphenyl ether and synthesizing method thereof
A technology for polybrominated diphenyl ethers and brominated diphenyl ethers, which are applied in the field of polybrominated diphenyl ethers and their synthesis, can solve the problems of difficult bromine substitution reaction, few bromine substitution digits, and no reports on the synthesis of high brominated OH/MeO-PBDEs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
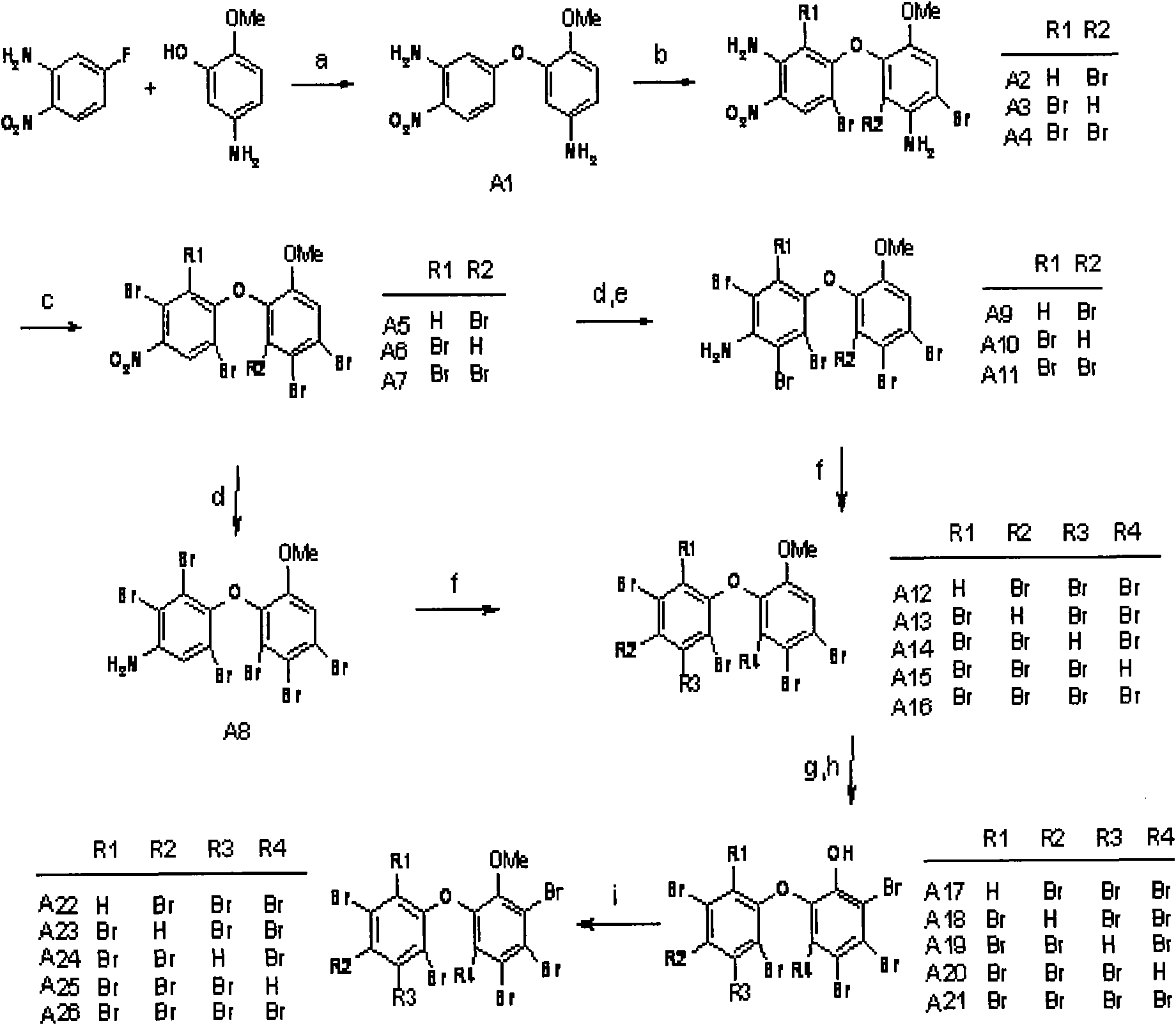
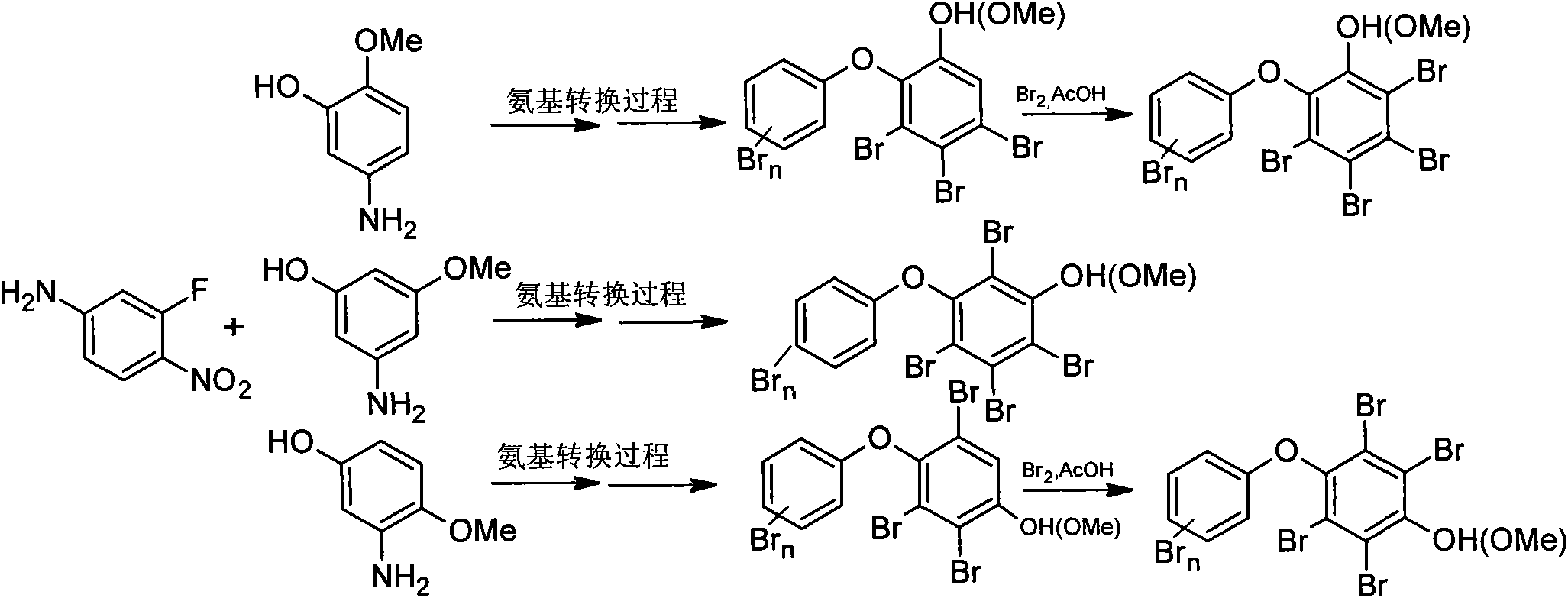
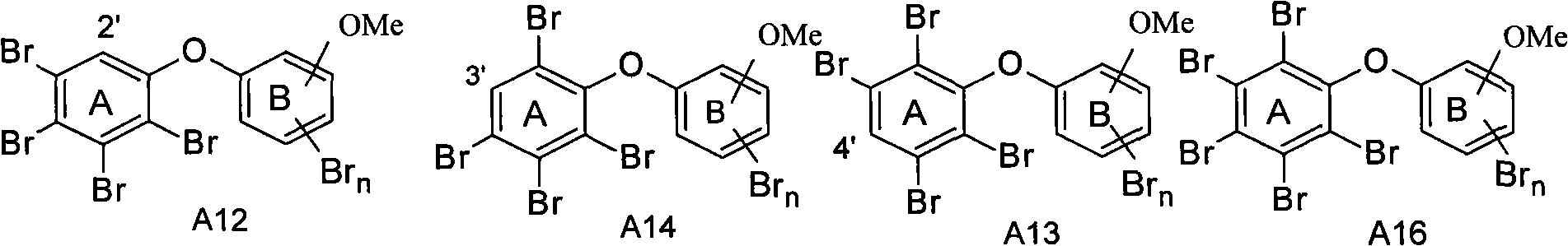
[0057] Embodiment one (taking the preparation process of A23 as example):
[0058] a. Taking 2-methoxy-5-aminophenol (1.39g, 10.0mmol) and 5-fluoro-2-nitroaniline (1.39g, 10.0mmol) as raw materials, in N,N-dimethylacetamide (DMAC, 15mL) as solvent and anhydrous Na 2 CO 3 (1.06g, 10.0mmol) was used as a catalyst for Williamson coupling reaction under the reaction conditions of 140°C, and the coupling product 5-(2-methoxy-4-aminophenol hydroxy)-2-nitroaniline A1 could be obtained after 3 hours of reaction. After the reaction solution was cooled, it was extracted with saturated brine and ethyl acetate, and the organic phase was extracted, washed with 1M NaOH (3×30ml), and then washed with distilled water (3×30ml). Column chromatography, the developing solvent is ethyl acetate / n-hexane 1:1, and drained to obtain a dark red solid. Yield: 1.80g, yield 65%; 1 H NMR: δ=8.08(d, J H4 =9.5Hz, 1H,H3), 6.32(dd, J H3,H6 =9.5, 2.5Hz, 1H, H4), 6.10(d, J H4 = 2.5Hz, 1H, H6), 6.86(d, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com