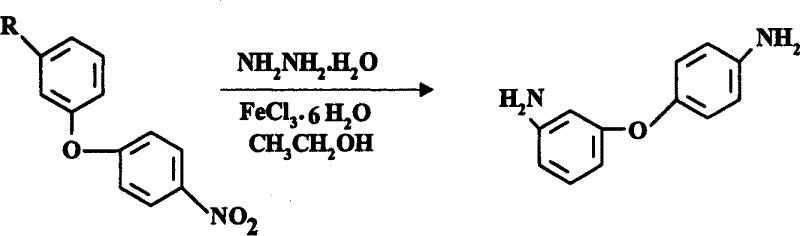
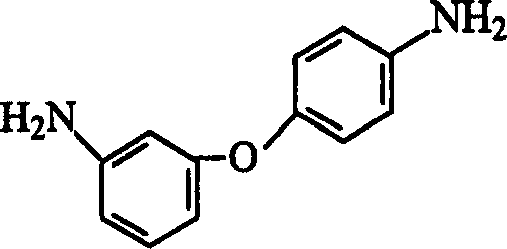
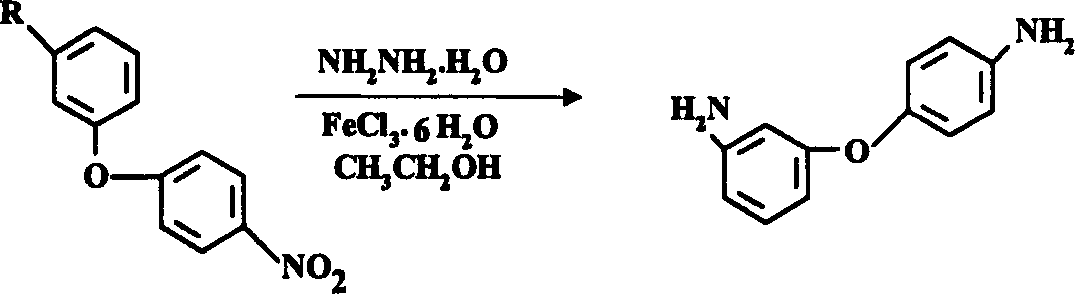
Process for preparing 3,4-diaminodiphenyl ether
A technology of diaminodiphenyl ether and nitrodiphenyl ether, which is applied to the preparation of amino hydroxyl compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of post-processing difficulties and unsafety, and achieve the goal of overcoming unsafe Factors, mild conditions, good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of 3,4'-diaminodiphenyl ether from 3-amino-4'-nitrodiphenyl ether:
[0025] Add 750ml of industrial ethanol to a 2000ml four-necked flask equipped with a reflux condenser, agitator, and a thermometer, and add 230g (1mol) of 3-amino-4'-nitrodiphenyl ether, 20g of activated carbon, and 0.2g of FeCl under stirring. 3 ·6H 2 O, introduce nitrogen, under nitrogen atmosphere, heat to 75±5°C, slowly drop in 155g 80% hydrazine hydrate (2.5mol) within 1 hour, keep reflux for 3 hours, cool to room temperature, filter, wash with 50ml industrial ethanol The filter residue was combined with ethanol, and 1000ml of water was added under stirring, and white crystals were precipitated. Filter, wash with water, and dry to obtain 186g of 3,4'-diaminodiphenyl ether with a yield of 93%, a purity of ≥99% (HPLC), and a melting point of 74.3-75.6°C (value in JP 61,221,159: 74-76°C).
Embodiment 2
[0027] Synthesis of 3,4'-diaminodiphenyl ether from 3,4'-dinitrodiphenyl ether:
[0028] Add 75ml of industrial ethanol in a 250ml four-neck flask equipped with a reflux condenser, agitator, and a thermometer, and add 26g (0.1mol) of 3,4'-dinitrodiphenyl ether, 2g of activated carbon, and 20mg of FeCl under stirring. 3 ·6H 2 O, introduce nitrogen, under nitrogen atmosphere, heat to 75±5°C under stirring, slowly drop in 31g of 80% hydrazine hydrate (0.5mol) within 1 hour, reflux for 2.5 hours after dropping, cool to room temperature, filter, use Wash the filter residue with 10ml of industrial ethanol, combine the ethanol, add 100ml of water under stirring, and precipitate white crystals. Filter, wash with water, and dry to obtain 17.8g of 3,4'-diaminodiphenyl ether with a yield of 89%, a purity of ≥99% (HPLC), and a melting point of 74.0-75.3°C.
Embodiment 3
[0030] Add 750ml of industrial ethanol to a 2000ml four-neck flask equipped with a reflux condenser, agitator, and a thermometer, and add 230g (1mol) of 3-amino-4'-nitrodiphenyl ether, 20g of activated carbon, and 0.2g of FeCl under stirring. 3 ·6H 2 O, introduce nitrogen, under nitrogen atmosphere, heat to 60±5°C, slowly drop in 155g 80% hydrazine hydrate (2.5mol) within 1 hour, reflux for 3 hours after dropping, cool to room temperature, filter, use 50ml industrial The filter residue was washed with ethanol, combined with ethanol, and 900ml of water was added under stirring to precipitate white crystals. Filter, wash with water, and dry to obtain 180 g of 3,4'-diaminodiphenyl ether with a yield of 90%, a purity of ≥99% (HPLC), and a melting point of 74.3-75.6°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com