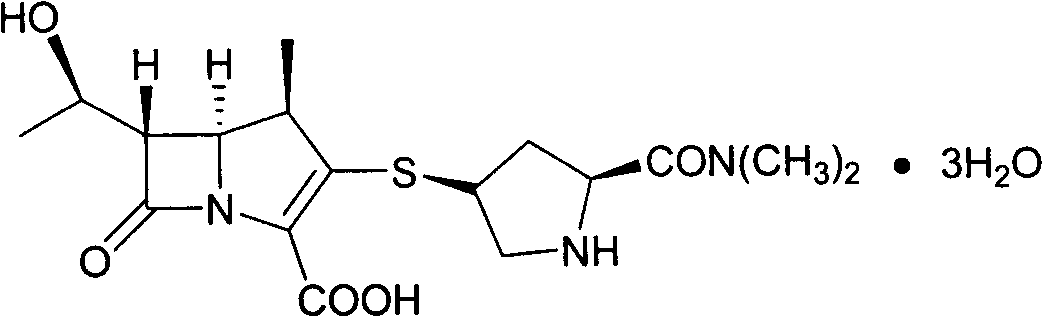
Preparation method of meropenem trihydrate crystal
A technology for meropenem trihydrate and meropenem, which is applied in the field of preparation of meropenem trihydrate crystals, can solve problems such as uninvolved solubility properties, and achieve the effects of good solubility, high purity, and rapid solubility properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under nitrogen atmosphere, distilled water (200ml) was heated to 45°C-50°C. The crude meropenem product (5.0 g, test = 93%) was added to distilled water, stirred at 45°C-50°C for 2 minutes, 1.0 g of activated carbon was added, and then rapidly cooled to 5°C-10°C. The carbon was filtered. Acetone (100ml) was added to the filtrate, the temperature was lowered to -20°C to -5°C, and the system was frozen. Vigorous stirring was maintained at this temperature for 30 minutes. Then the temperature of the system was raised to 5°C-20°C, at which temperature acetone (300ml) was added. Then lower the temperature to 0°C to 5°C and stir for 3 to 4 hours. The crystalline solid was filtered, washed with acetone, and dried under reduced pressure at 40° C. for 3-4 hours to obtain white meropenem trihydrate crystals (4.03 g, 99.5% assay). In the clarity test, the time for no turbidity is 30 seconds, the time for less than 5 particles is 45 seconds, and the time for complete dissolutio...
Embodiment 2
[0031] The method of Example 1 was repeated using crude meropenem (5.0 g, test=80%) to obtain white meropenem trihydrate crystals (3.52 g, test 98.2%). In the clarity test, the time for no turbidity is 30 seconds, the time for less than 5 particles is 60 seconds, and the time for complete dissolution is 75 seconds.
Embodiment 3
[0033] Distilled water (100 ml) was heated to 65°C under nitrogen atmosphere. The crude meropenem product (5.0 g, inspection = 93%) was added to distilled water, stirred at 65°C for 2 minutes, 1.0 g of activated carbon was added, and then rapidly cooled to 5°C-10°C. The carbon was filtered. Acetone (50ml) was added to the filtrate, the temperature was lowered to -20°C to -5°C, and the system was frozen. Vigorous stirring was maintained at this temperature for 30 minutes. Then the temperature of the system was raised to 5°C-20°C, at which temperature acetone (150ml) was added. Then lower the temperature to 0°C to 5°C and stir for 3 to 4 hours. The crystalline solid was filtered, washed with acetone, and dried under reduced pressure at 40° C. for 3-4 hours to obtain white meropenem trihydrate crystals (4.15 g, 99.3% assay). In the clarity test, the time for no turbidity is 30 seconds, the time for less than 5 particles is 45 seconds, and the time for complete dissolution is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com