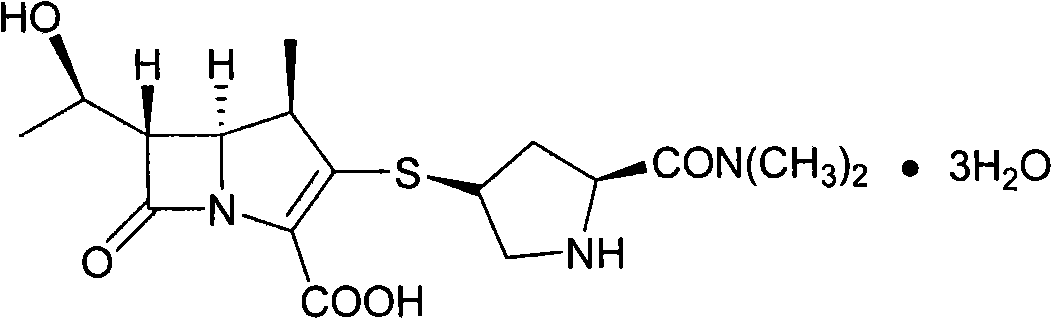
Preparation method of Meropenem trihydrate crystals
A technology of meropenem and trihydrate, which is applied in the direction of organic chemistry, can solve the problem of reducing the solvent residue of meropenem trihydrate that is difficult to achieve and has not been seen, and achieve the effect of less raw material loss and simple and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under nitrogen atmosphere, heat 200ml of distilled water to 65°C-70°C. Add 10.0 g of the crude meropenem product (test = 92%) into distilled water, stir at 65°C-70°C for 2 minutes, add 1.0 g of activated carbon, then rapidly cool to 10°C-15°C, remove the activated carbon by filtration, and cool the filtrate to At about 10°C, add a small amount of seed crystals, stir at a relatively fast speed for 4 hours, keep the temperature at 2-5°C, and filter to obtain 6.3 g of meropenem trihydrate. The related substances of the obtained crystals are measured by the Pharmacopoeia method, and the single impurity is less than 0.1%, the total impurity is less than 0.3%, and the residue of various organic solvents is less than 0.5‰. Add 400ml of acetone to the mother liquor, keep stirring at 2-5°C for 1 hour, then start to add 400ml of acetone dropwise, finish dropping after 30 minutes, keep stirring at this temperature for 2 hours, then filter, and recover 2.3g of meropenem.
Embodiment 2
[0025] Under nitrogen atmosphere, heat 200ml of distilled water to 65°C-70°C. Add 10.0 g of the crude meropenem product (test = 92%) into distilled water, stir at 65°C-70°C for 2 minutes, add 1.0 g of activated carbon, then rapidly cool to 10°C-15°C, remove the activated carbon by filtration, and cool the filtrate to 10°C, without adding seed crystals, stirred at a relatively fast speed for 4 hours, kept the temperature at 2-5°C, and filtered to obtain 5.4 g of meropenem trihydrate crystals. The related substances of the obtained crystals are measured by the Pharmacopoeia method, and the single impurity is less than 0.1%, the total impurity is less than 0.3%, and the residue of various organic solvents is less than 0.5‰. Add 400ml of acetone to the mother liquor, keep stirring at 2-5°C for 1 hour, then start to add 400ml of acetone dropwise, finish dropping after 30 minutes, keep stirring at this temperature for 2 hours, then filter, and recover 3.1g of meropenem.
Embodiment 3
[0027] Under nitrogen atmosphere, 300ml of distilled water was heated to 50°C-55°C. Add 10.0 g of the crude meropenem product (test = 92%) into distilled water, stir at 50°C-55°C for 2 minutes, add 1.0 g of activated carbon, then rapidly cool to 10°C-15°C, remove the activated carbon by filtration, and cool the filtrate to At 10°C, add a small amount of seed crystals, stir at a relatively fast speed for 4 hours, keep the temperature at 2-5°C, and filter to obtain 5.6 g of meropenem trihydrate crystals. The related substances of the obtained crystals are measured by the Pharmacopoeia method, and the single impurity is less than 0.1%, the total impurity is less than 0.3%, and the residue of various organic solvents is less than 0.5‰. Add 600ml of acetone to the mother liquor, keep stirring at 2-5°C for 1 hour, then start to add 600ml of acetone dropwise, finish dropping after 30 minutes, keep stirring at this temperature for 2 hours, then filter, and recover 2.4g of meropenem. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com