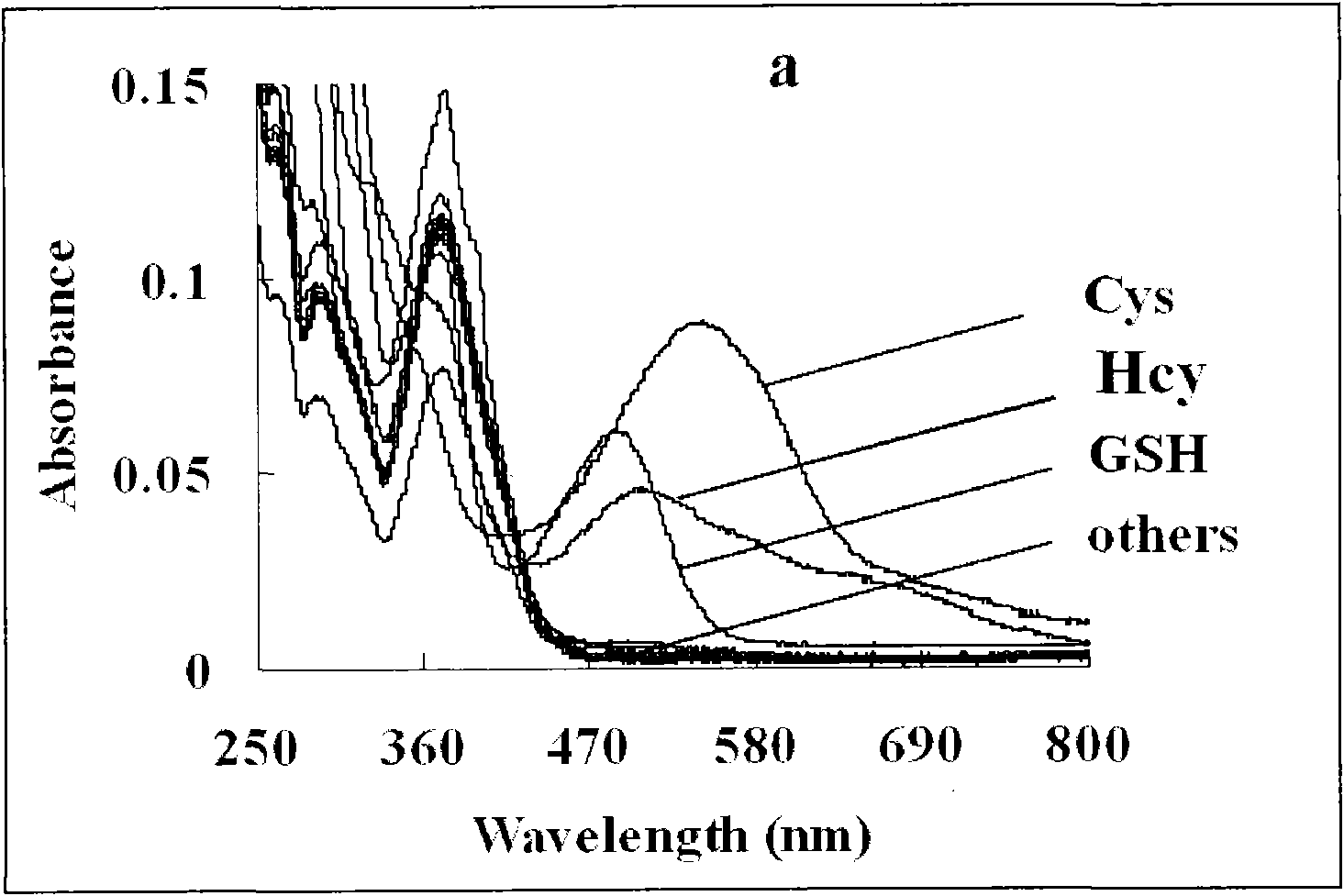
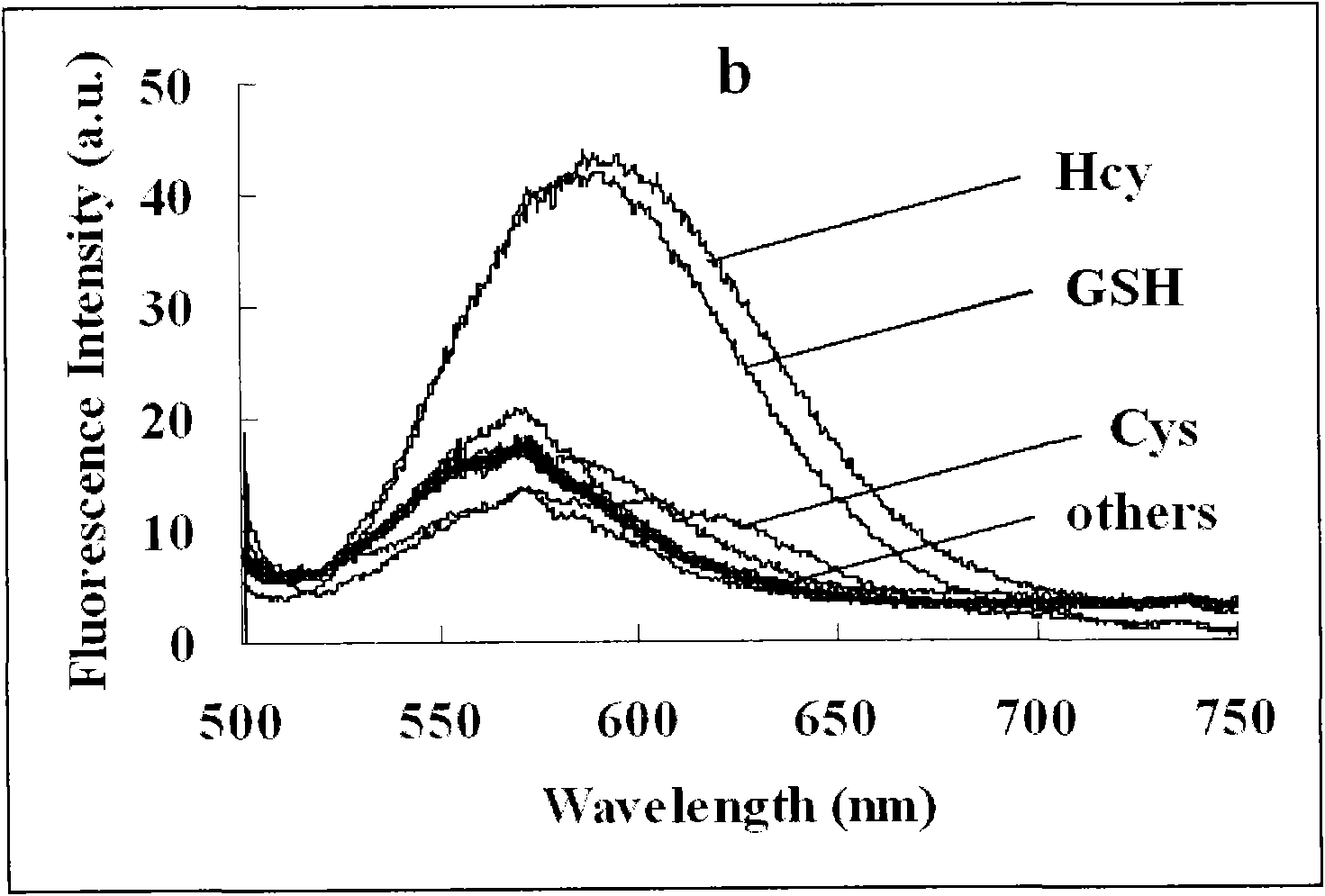
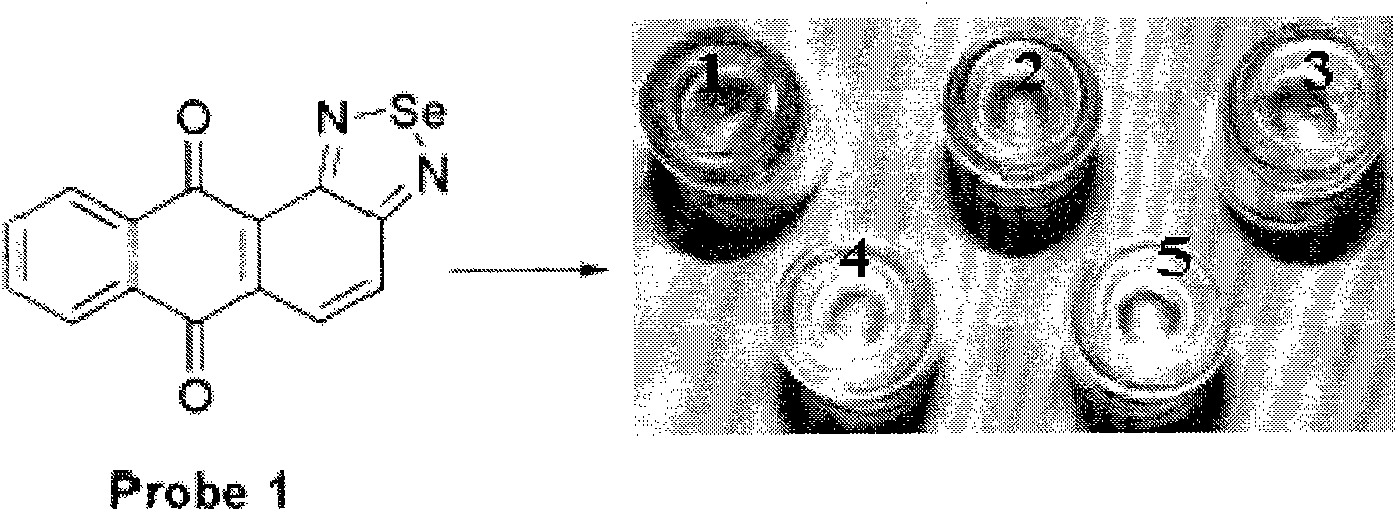
Colorimetric fluorescence probe for high selectivity multiple biological thiol and preparation method thereof
A high-selectivity, fluorescent probe technology, applied in the field of life sciences, can solve the problems of inability to distinguish and identify the contents of cysteine, homocysteine and glutathione, and achieve low cost and simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0030] 238.2mg (1mmol) 1,2-diaminoanthraquinone and 221.9mg (2mmol) selenium dioxide were ground respectively in an agate mortar, then the two compounds after grinding were mixed, continued to grind for 1h, and then The ground mixture was dissolved in chloroform, filtered, the solvent was evaporated under reduced pressure, the residue was separated by column chromatography, chloroform was used as eluent, and then the eluent was removed from chloroform eluent to obtain the target The pure product is 261.8 mg, the yield is 84%, and the reaction formula is as follows:
[0031]
[0032] The H NMR spectrum and high-resolution mass spectrometry characterization data of the target substance obtained are as follows:
[0033] 1 H-NMR (400MHz, DMSO-d 6 )δ(*10 -6 ): 7.95(t, J=7.0Hz, 2H), 8.20(d, J=7.6Hz, 2H), 8.34(d, J=2.8Hz, 2H);
[0034] HRMS (ESI positive): [M+H] + calcd for C 14 h 7 N 2 o 2 Se 314.96676, found 314.96644.
[0035] Identify and analyze the obtained target ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com