Triterpenoid saponin compound, application and preparation method
A technology of triterpene saponins and compounds, applied in the field of triterpene saponins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
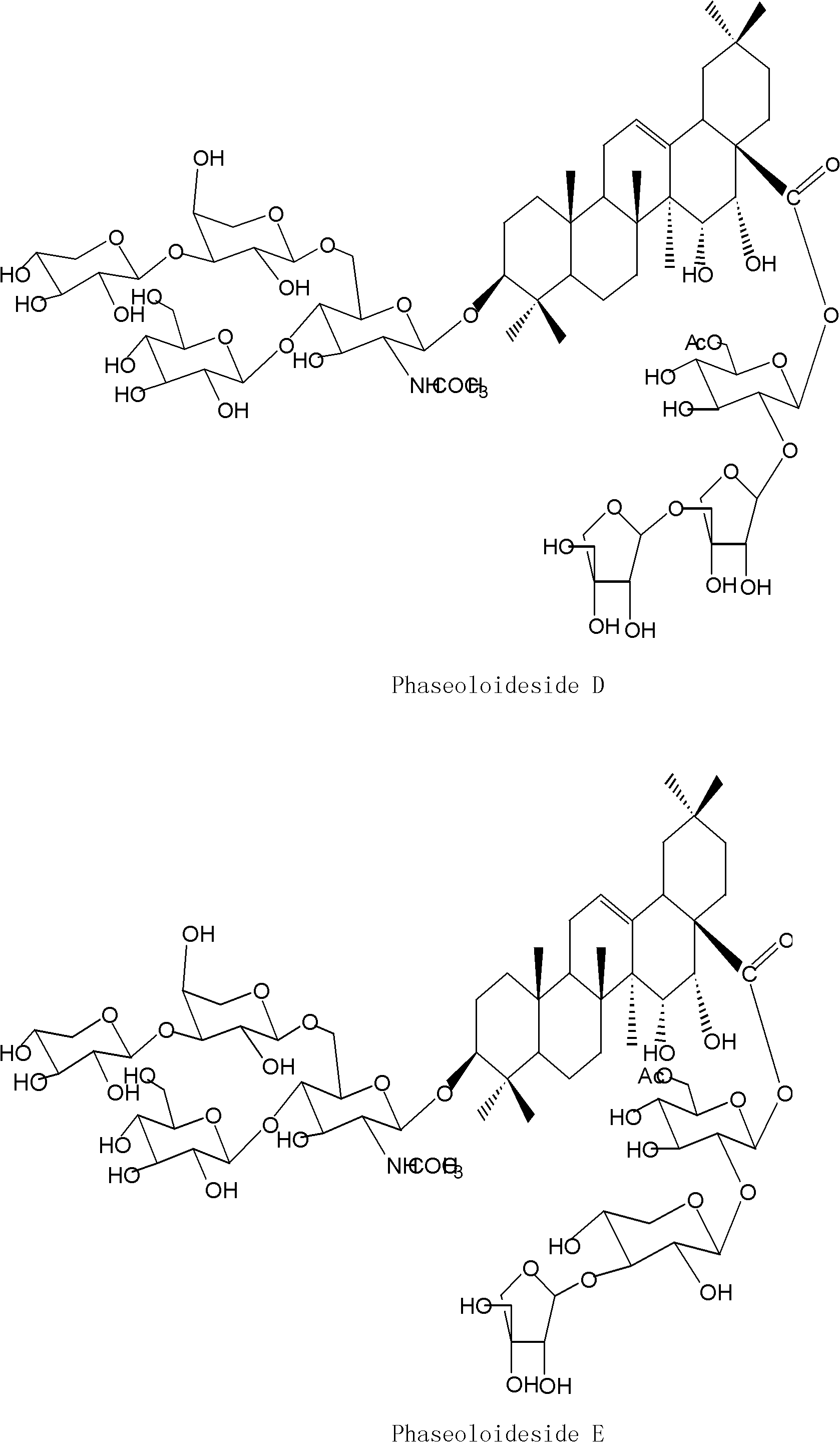
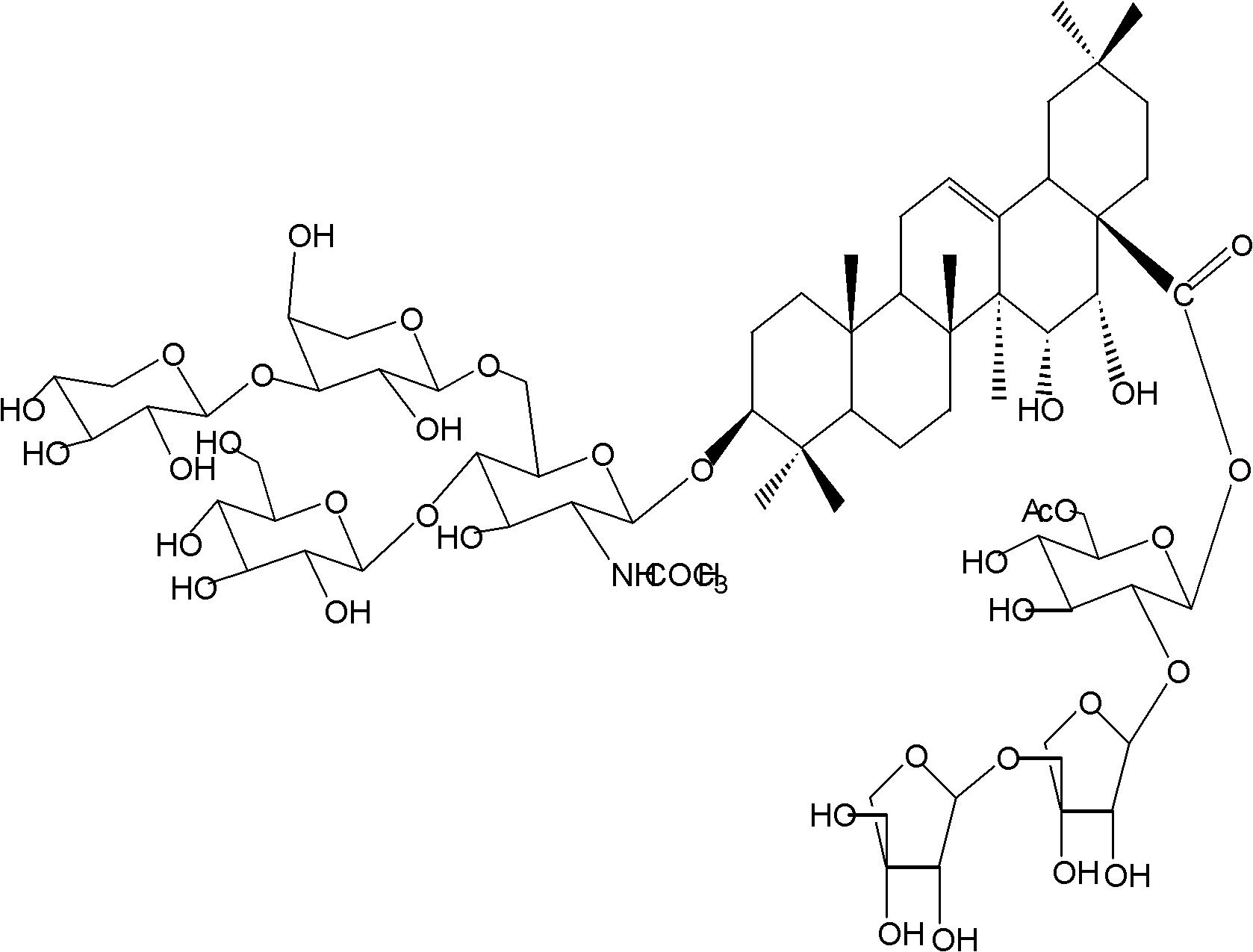
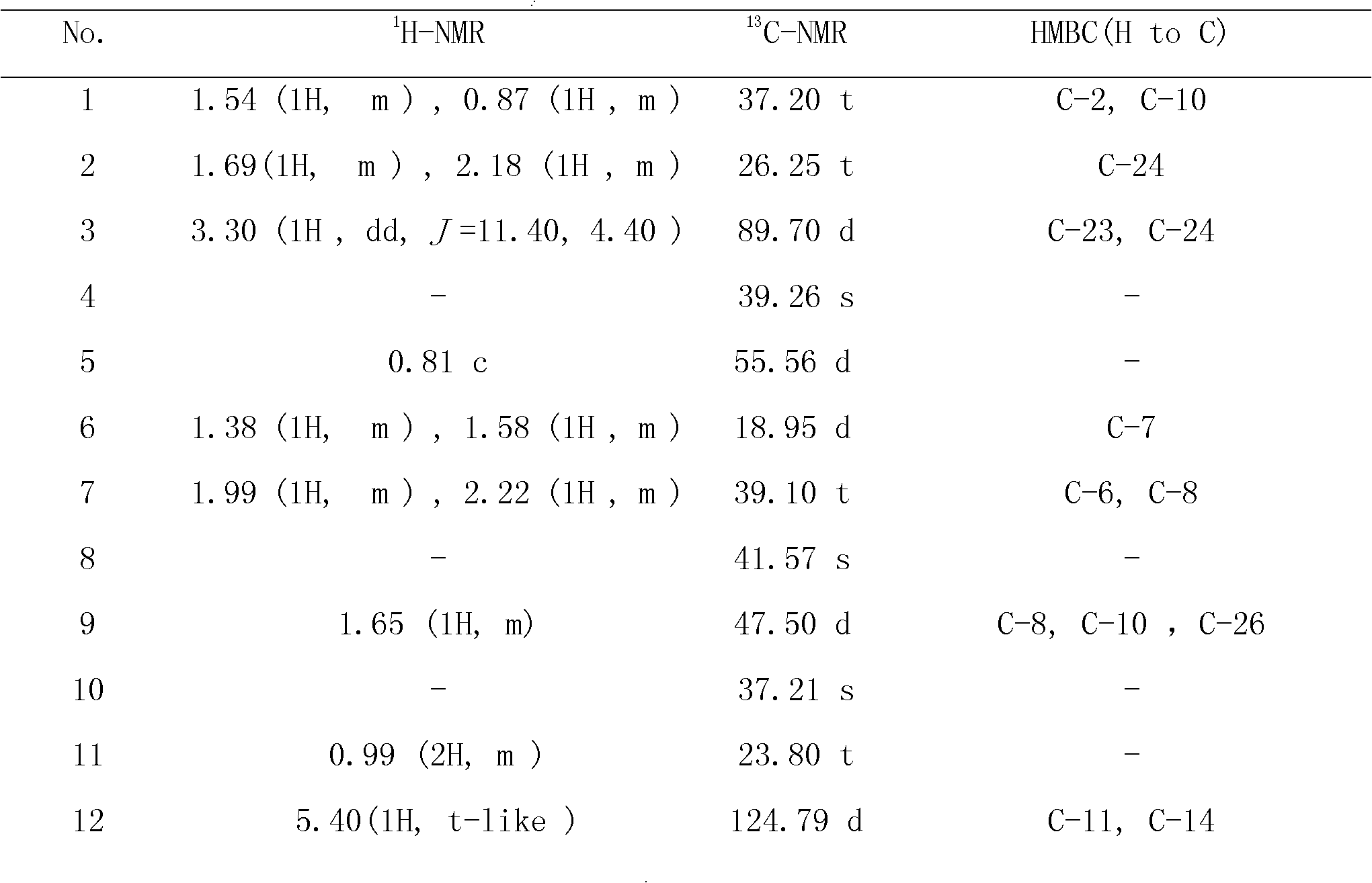
[0026] Example 1 Preparation of Triterpenoid Saponins Phaseoloideside D and Phaseoloideside E
[0027] 1. Extraction, separation and purification methods
[0028] Shell the dried twig fruit medicinal material to obtain the twig twig kernel, dry it in the sun, crush and weigh 8000 grams, then soak and extract with 4 times the amount of 70% ethanol at room temperature for 3 times, each time for 24 hours, and concentrate the extract under reduced pressure to obtain semi Transparent extract 518g. Dissolve the extract in a methanol-water mixed solvent with a volume ratio of 9:1, repeatedly extract with an equal volume of petroleum ether until the petroleum ether layer is basically colorless, dissolve the extract in water, and repeatedly extract with an equal volume of ethyl acetate The ethyl acetate layer was basically colorless, and then extracted repeatedly with an equal volume of n-butanol until the n-butanol layer was basically colorless, and the n-butanol layers were combined...
Embodiment 2 3
[0047] Example 2 Preparation of Triterpenoid Saponins Phaseoloideside D and Phaseoloideside E
[0048] Sun-dried the twig seed kernels, crushed and weighed 10,000 grams, leached 7 times with 5 times the amount of 70% ethanol at room temperature, 24 hours each time, concentrated the extract, and then degreased with petroleum ether, and the degreased extract was normalized After alcohol extraction, use ethanol-soluble aqueous layer components, dry under vacuum (dry weight 345 g), redissolve in methanol-water solution with a volume ratio of 9:1, pass through macroporous resin, and elute with water and ethanol gradient , collect 50% ethanol eluate, reclaim ethanol under reduced pressure, obtain 61.61 grams of total saponin extract. The total saponin extract was subjected to silica gel column chromatography, using ethyl acetate: methanol: water as the eluent gradient elution, collecting the fractions containing the triterpene saponins, and going through ODS column chromatography, u...
Embodiment 3
[0049] Example 3 Effects of Phaseoloideside D and Phaseoloideside E on Tumor Cells
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com