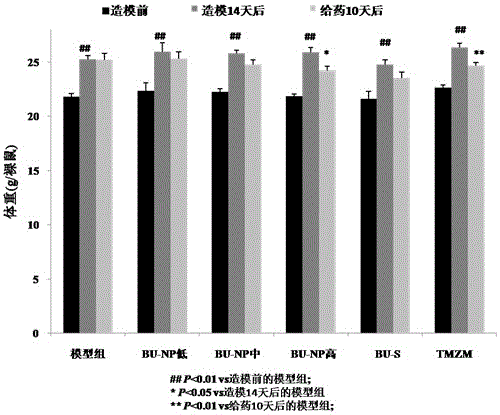
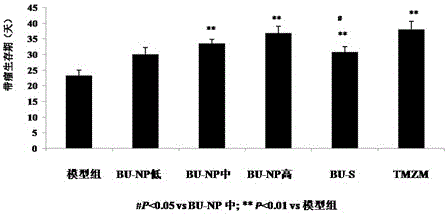
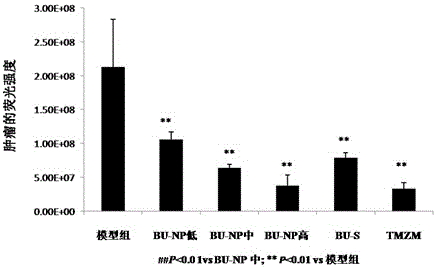
Application of bufotoxin extract in preparation of medicine for treating human brain glioma
A human brain glioma and extract technology, applied in the field of medicine, can solve the problems of killing human brain glioma cells, easy to recur, difficult to completely resection, etc., to prolong the survival period of tumors, improve brain targeting, The effect of a good dose-response relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0032] Experimental example 1 Separation and purification of high-purity bufoginin extract
[0033] Crush the toad venom medicinal material and beat it into a coarse powder with a pulverizer, pass through a 60-mesh sieve, weigh 200g, add 2L of ethanol with a volume concentration of 80%, heat and reflux in a water bath for 2 hours, place it at room temperature, suction filter, and evaporate the filtrate to dryness to obtain crude Extract.
[0034] Weigh 20 g of the crude extract of Bufoni venom, add 200 mL of distilled water, ultrasonically suspend, then add 200 mL of ethyl acetate to repeat the extraction 3 times, combine all the ethyl acetate layers and concentrate to dryness under reduced pressure to obtain the extract. Dissolve the extract in a chloroform-methanol mixture with a volume ratio of 1:1, wherein the quality of the chloroform-methanol mixture is 1.5 times the mass of the extract, filter off the insolubles to obtain a clear solution; add the clear solution while a...
experiment example 2
[0036] Experimental Example 2 Preparation of Bufagenin Nanostructured Lipid Carrier
[0037] Prescription: (by weight ratio) In this experimental example, the extract of toad venom prepared in Experimental Example 1 was used.
[0038]
[0039] Preparation process: Weigh the prescribed amount of lipid material and toad venom extract, put them in a 75°C water bath and heat them with magnetic stirring to dissolve, and obtain a transparent and uniform oil phase; add the prescribed amount of LipoidE80?, PluronicF68 and sodium deoxycholate to an appropriate amount of water for injection , heated to 75°C under 500rpm magnetic stirring, dispersed and dissolved to obtain the water phase; under magnetic stirring, the water phase was added dropwise to the oil phase at the same temperature while it was hot, magnetically stirred for 5 minutes, then ultrasonically dispersed for 10 minutes, cooled in an ice-water bath under stirring conditions, Under sterile conditions, sterilize by filtr...
experiment example 3
[0040] Preparation of Experimental Example 3 Bufagenin Submicroemulsion Preparation
[0041] Prescription: (by weight ratio) In this experimental example, the extract of toad venom prepared in Experimental Example 1 was used.
[0042]
[0043] Preparation process: Weigh the prescribed amount of medium-chain triacylglycerol and long-chain triacylglycerol, add LipoidE80? and toad venom extract in sequence, heat and stir at 75°C for 30 minutes until dissolved, and obtain a clear drug-containing oil phase; PluronicF68, sodium oleate and glycerin were added to an appropriate amount of water for injection, heated and stirred at 70°C to dissolve to obtain the water phase; under the stirring of a high-speed disperser at 12,000 rpm, the oil phase was slowly added to the water phase, and continued to stir for 3 minutes to prepare to obtain colostrum; colostrum with 0.1mol L -1 Adjust the pH value of hydrochloric acid solution to 6.0, dilute to 100mL with water for injection, cool, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com