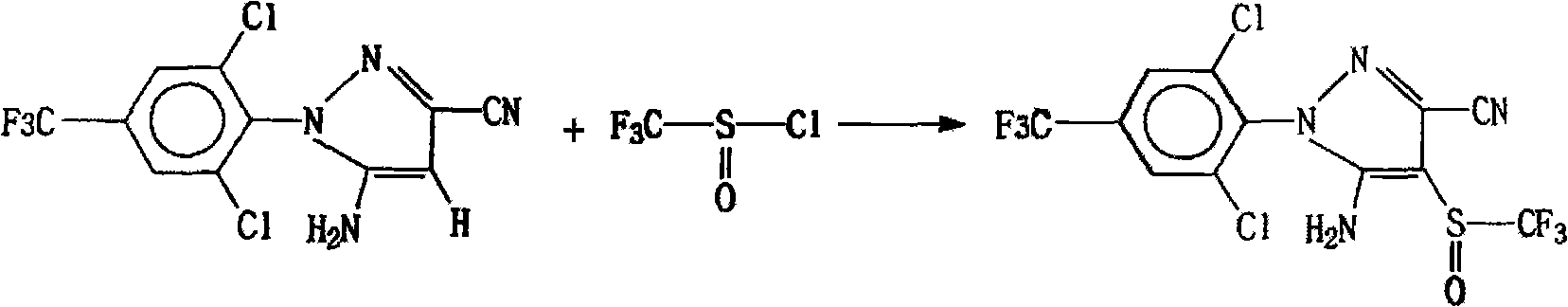
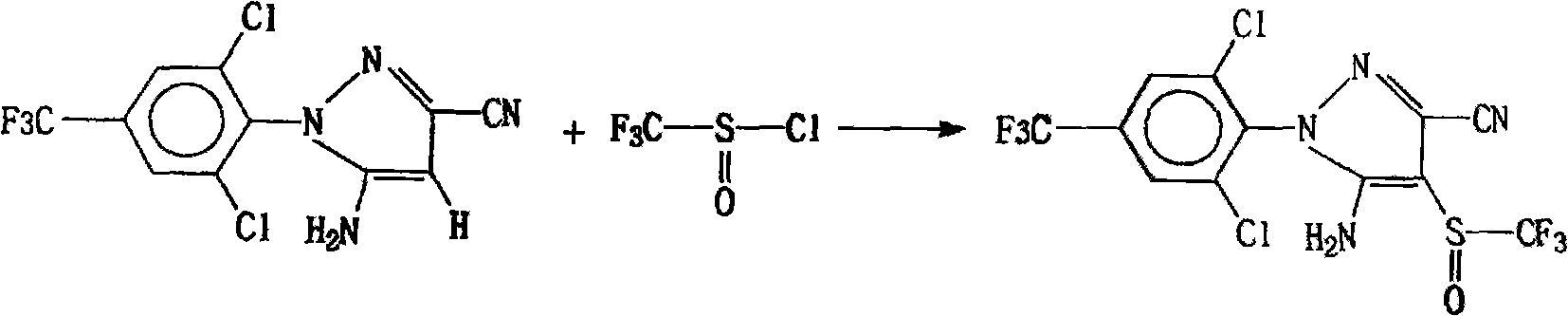Method for synthesizing and purifying fipronil
A purification method and technology of fipronil, which is applied in the field of preparation of high-efficiency insecticide fipronil, can solve the problems of high toxicity of trifluoromethylthiohaloalkane, difficult operation process, high production cost, etc., and achieve excellent product quality and excellent process The effect of simple conditions and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0013] In a four-neck flask with an electric stirrer, a thermometer, and a reflux condenser, and the flask is placed in a container that can hold ice water, 29.0 grams (0.22 moles) of thionyl chloride and 250 ml of anhydrous benzene liquid are first dropped into, cooled And keeping the temperature at 0°C, add 45.0 g (0.5 moles) of CF in 3 times 3 SO 2 Cl, the reaction temperature is controlled at 0-5°C, react until no gas overflows, heat up to 15-20°C and keep warm for 8 hours; then add 50.0 grams (0.16 moles) of 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl) pyrazole and 52.8 grams (0.17 moles) of p-toluenesulfonic acid dimethylamine salt mixture, while adding 1 gram of catalyst, the controlled temperature is 15 ° C, stirred for 0.5 hours, Then the temperature was raised to 35-45° C. to react for 12 hours, and the solvent was evaporated under reduced pressure to obtain a solid reactant; the obtained solid was washed with water and filtered, and the filter cake was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com