Recombinant human interleukin-1 receptor antagonist in-situ gel
A receptor antagonist and in situ gel technology, applied in the field of medicine, can solve problems such as inaccurate dosage, poor fluidity, difficulty in meeting the needs and recognition of the market and patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Recombinant human interleukin-1 receptor antagonist in situ gel No. 1
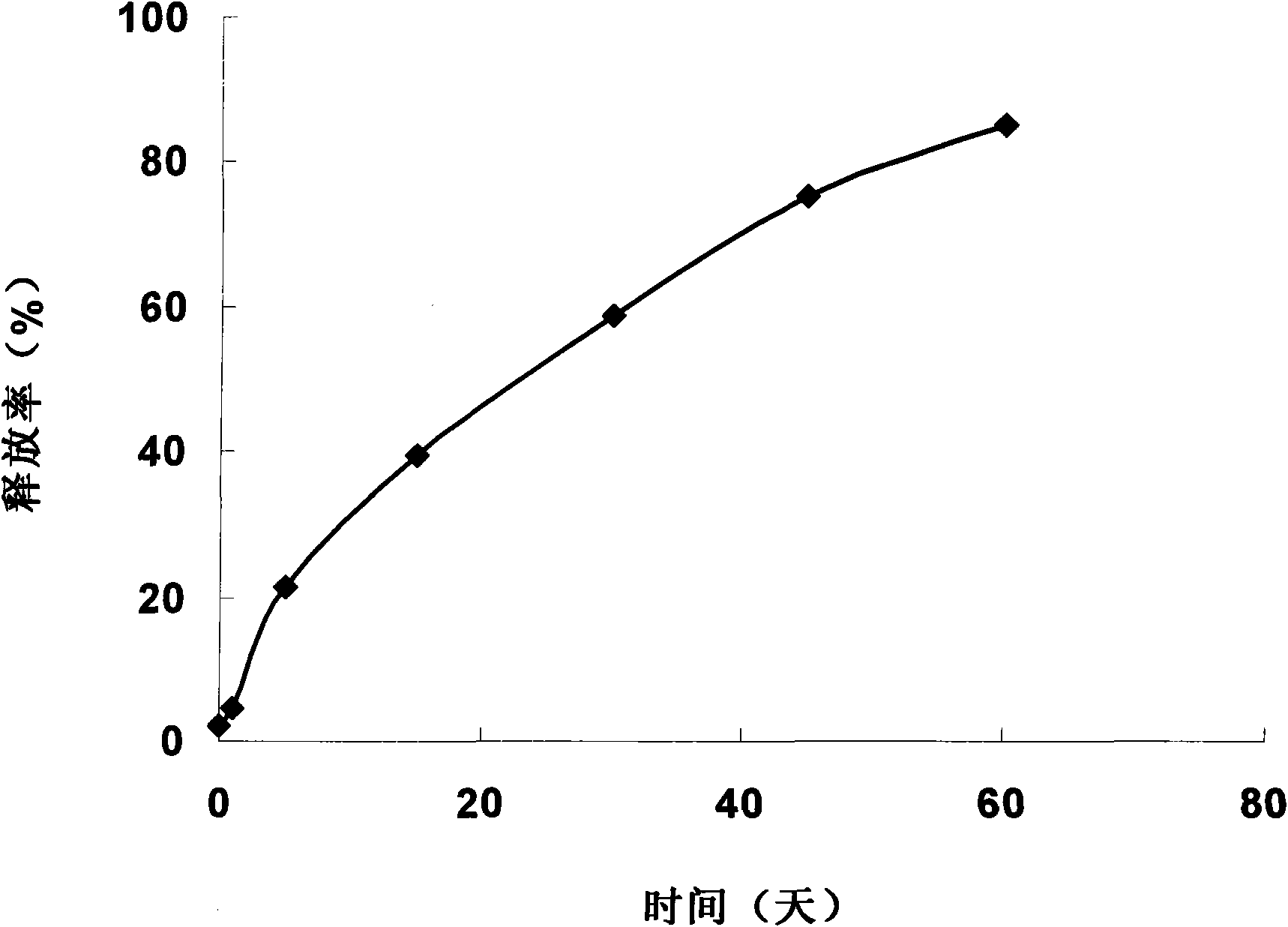
[0025] Take 15g of poloxamer 407, 15g of poloxamer 188, and 0.1g of gellan gum, add 50mL of boric acid buffer to fully swell into a uniform solution. Add 3mL IL-1ra receptor antagonist stock solution and 0.3g chlorobutanol. Mix evenly and divide for use. The in situ gel has dual functions of temperature sensitivity and ion sensitivity, and is a flowing liquid at room temperature ( figure 1 ), 5min at 37°C to form a gel ( figure 2 ). The in situ gel can be used in various administration routes and modes mentioned in the specification.
Embodiment 2
[0026] Example 2. Recombinant human interleukin-1 receptor antagonist in situ gel No. 2
[0027] Weigh 10 g of polyethylene glycol monomethyl ether / polylactic acid-glycolic acid copolymer (mPEG-PLGA) and 3 g of carbomer in 50 mL of water, stir and place it in a refrigerator at 4 °C with continuous shaking until mPEG- After PLGA swells, shake well to make a homogeneous solution. Add 3mL IL-1ra receptor antagonist stock solution, mix evenly, and dispense for use. The in-situ gel has dual effects of pH sensitivity and temperature sensitivity, and is a flowing liquid at room temperature, and becomes a gel within 5 minutes after intranasal administration. The in situ gel can be used in various administration routes and modes mentioned in the specification.
Embodiment 3
[0028] Example 3. Recombinant human interleukin-1 receptor antagonist in situ gel No. 3
[0029] Take 15g of poloxamer 407 and 15g of poloxamer 188, 0.1g of gellan gum, 2.5g of chitosan and add 50mL of water to fully swell to form a uniform solution. Add 3mL IL-1ra receptor antagonist stock solution, mix evenly, and dispense for use. The in-situ gel has dual functions of temperature sensitivity and ion sensitivity. At the same time, chitosan acts as an absorption accelerator. It is a flowing liquid at room temperature and forms a gel within 5 minutes after spraying into the nasal cavity. The in situ gel can be used in various administration routes and modes mentioned in the specification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com