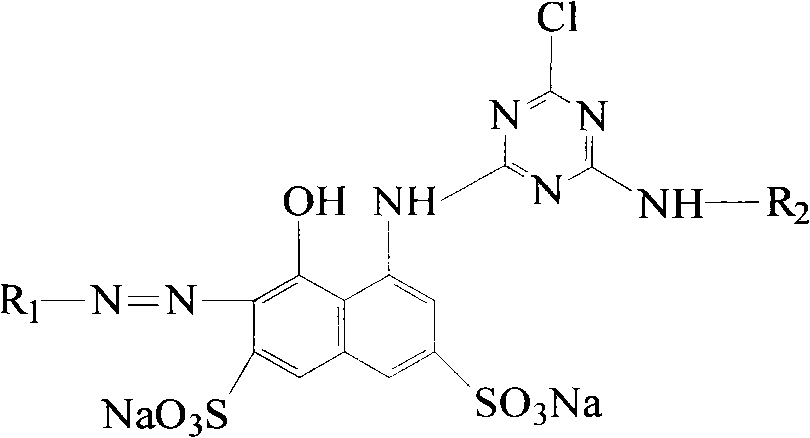
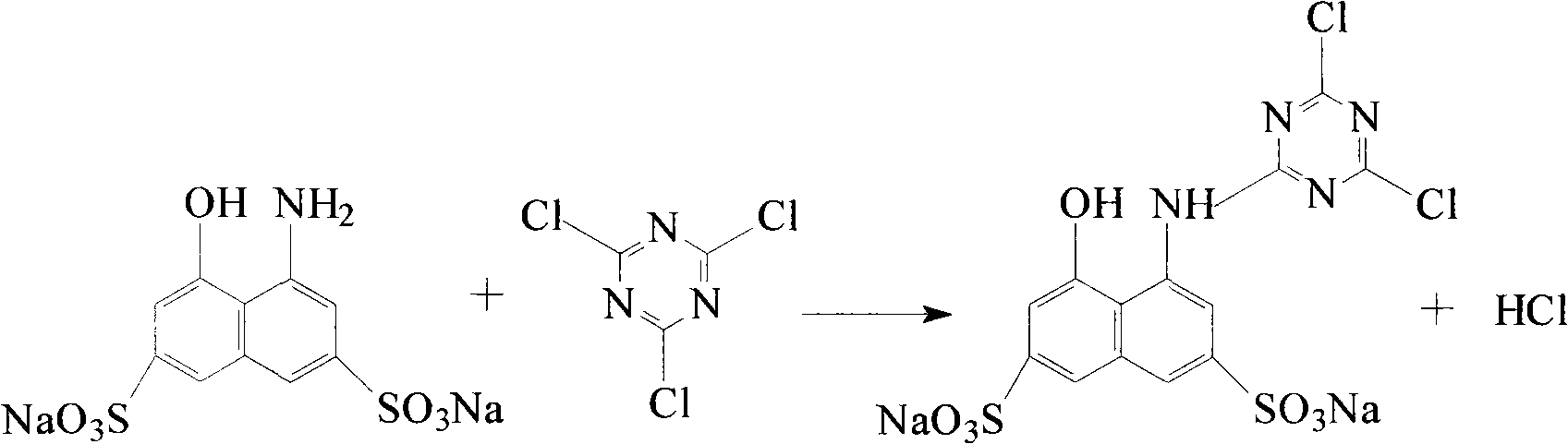
K-type active light-resistant red azo dye and preparation method thereof
An azo dye and reactive technology, which is applied in the field of K-type reactive lightfast red azo dye, can solve the problems of low dye utilization rate, low wet rubbing fastness of dyed fabrics, low light fastness to sunlight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1, the synthesis of reactive light fast red KA
[0011] 1. Preparation of cyanuric chloride and H acid condensation reaction solution
[0012] In a 250mL four-necked flask equipped with a stirrer, dropping funnel and thermometer, respectively add 15g of crushed ice, 10mL of water and 0.011mol of cyanuric chloride for beating at 0°C for 30 minutes, then dropwise add pH=7 within 1 hour ~8 H acid solution with a H acid content of 0.01mol. After the addition, keep the pH value of the reaction solution at 2~3, continue to stir and react at 5~8°C for 3 hours, and use an amino indicator to detect the presence of H acid in the solution. Afterwards, stop the reaction and filter to obtain a clarified condensate for later use. The reaction formula is as follows:
[0013]
[0014] 2. Preparation of p-aminobenzenesulfonic acid diazonium salt solution
[0015] Add 10mL of water and 0.01mol of p-aminobenzenesulfonic acid into a 100mL beaker, start stirring, neutralize...
Embodiment 2
[0023] Embodiment 2, the synthesis of active photostable red KB
[0024] The molecular structure formula of reactive photostable red KB is as follows:
[0025]
[0026] The preparation process of active light fast red KB is the same as embodiment 1, just the p-aminobenzenesulfonic acid in embodiment 1 is replaced with m-aminobenzenesulfonic acid; 4-(4-aminobenzoate group)-2,2, Reactive photostable red KB can be obtained by replacing 6,6-tetramethylpiperidine with 4-(4-aminobenzamido)-1,2,2,6,6-pentamethylpiperidine.
Embodiment 3
[0027] Embodiment 3, the synthesis of reactive light fast red KC
[0028] 1. Preparation of cyanuric chloride and H acid condensation reaction solution
[0029] The preparation process of cyanuric chloride and H acid condensation reaction liquid is with embodiment 1.
[0030] 2, Preparation of 4-(4-aminobenzoate group)-2,2,6,6-tetramethylpiperidine diazonium salt solution
[0031] Add 30mL of water, 0.01mol of 4-(4-aminobenzoate)-2,2,6,6-tetramethylpiperidine, 3.5mL of 30% hydrochloric acid into a 150mL beaker, and start stirring to make the amino compound completely Dissolve and cool to 0°C in an ice-water bath. Dissolve 0.0105 mol of sodium nitrite in 2 mL of water, cool to 0°C in an ice-water bath, quickly add to the amino compound, keep the pH of the reaction solution below 3 after the addition, and continue to stir the reaction at a temperature lower than 5°C, after 2 hours The diazotization reaction is complete, and now the reaction solution is blue to the Congo red t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com