Long-acting interferon fusion protein and application thereof
A technology of fusion protein and interferon, applied in the direction of interferon, prolonging plasma life fusion, cytokine/lymphokine/interferon, etc., can solve the problem of no interferon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
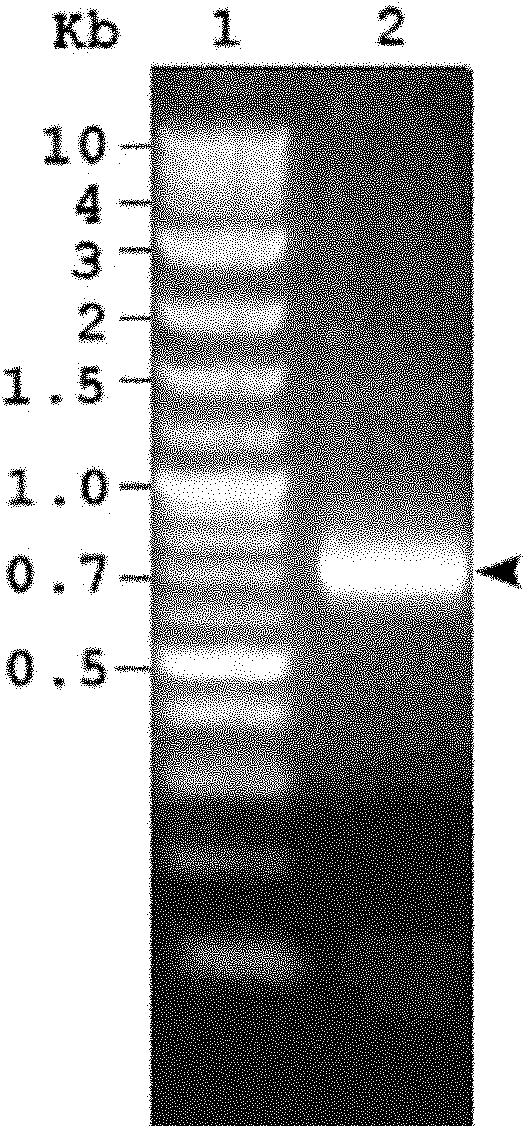
[0038] Example 1 Obtaining of recombinant cIFN-ABP protein coding gene and construction of high-efficiency expression engineering bacteria
[0039] The cIFN coding gene sequence was synthesized artificially and cloned into the pBAD18 plasmid vector (purchased from Invitrogen, Carlsbad, USA, its sequence is referred to J. Bacteriol. 177, 4121-4130 (1995)) to obtain pBAD-cIFN.
[0040] First, using the cIFN coding sequence in the plasmid as a template, the human albumin-specific affinity short peptide (HSA-BP, ABP for short) coding sequence and linking sequence are added to its C-terminus by PCR. The pBAD18 plasmid is a high-efficiency expression plasmid in Escherichia coli. The cIFN coding sequence was cloned into the XbaI and SacI restriction sites of this plasmid. Therefore, the coding sequence of ABP, connecting peptide and restriction site was added in 2 steps by PCR method so that the cIFN-ABP fusion protein coding sequence could be re-cloned into the pBAD18 expression ve...
Embodiment 2
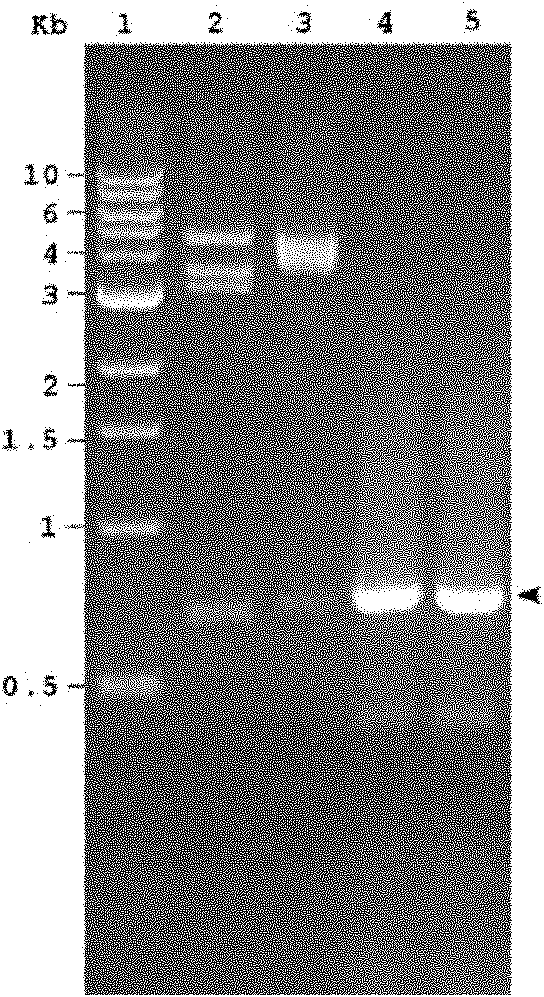
[0079] Example 2. High-efficiency expression and purification of rcIFN-ABP protein
[0080] TOP10 competent bacteria were purchased from Invitrogen, USA. The pBAD-IFNm plasmid was transformed into TOP10 bacterial cells according to conventional methods, and the transformed bacteria were inoculated on LB agarose culture plates (containing 100 μg / ml ampicillin), and cultured overnight at 37°C. Take a single colony and inoculate it in 5ml LB liquid medium (containing 100μgml ampicillin), culture it in a shaker at 37°C (225-250RPM) overnight. Take 1ml of the overnight bacterial culture and add it to 100ml LB liquid medium (containing 100μg / ml ampicillin), culture it on a shaker at 37 degrees, when OD600=~0.5, add 1ml 20% L-arabinose, and continue to cultivate for 4 -8 hours. The induced bacterial cells were harvested, and the bacterial culture was centrifuged in a Sorvall centrifuge (RC-5C, Dupont, USA, rotor model SS-34) at 4° C., 6000 RPM for 15 minutes. The bacterial pellet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com