Method for preparing 2-(5-fluoro-2,4-dinitrophenoxy) acetic acid and ester thereof
A technology of dinitrophenoxy and dinitrobenzene, which is applied in the field of preparation of 2-acetic acid and its esters, can solve problems such as complicated process, low reaction conversion rate and product yield, and high difficulty in industrialization, and achieves reaction yield. The effect of high efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
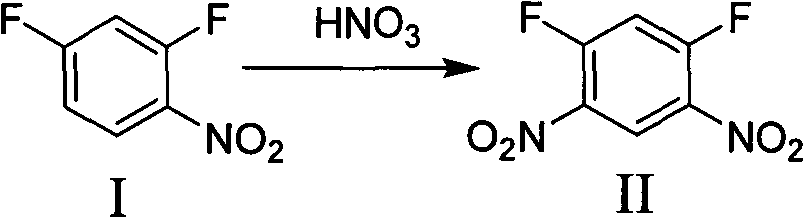
[0027] Preparation of 1,5-difluoro-2,4-dinitrobenzene:
[0028] Add 2,4-difluoronitrobenzene (320g, 2mol) into a 2000mL reaction flask, add 600mL of concentrated sulfuric acid dropwise at room temperature, complete the dropwise addition within 2 hours, start to add 250mL of fuming nitric acid dropwise, add dropwise for 4 hours, add dropwise complete. Insulated at 90°C for 2 hours, poured into ice, filtered, and dried to obtain 400 g (yield 95%) of the title compound, the content of which was greater than 97%.
[0029] 1 H NMR (500Hz, CDCl 3 ): δ8.99(t, 1H, J=7.5Hz), 7.34(t, 1H, J=10Hz)
Embodiment 2
[0031] Preparation of 1,5-difluoro-2,4-dinitrobenzene:
[0032] Add 2,4-difluoronitrobenzene (320g, 2mol) into a 2000mL reaction flask, add 600mL of concentrated sulfuric acid dropwise at room temperature, complete the dropwise addition within 2 hours, start to add 250mL of fuming nitric acid dropwise, add dropwise for 4 hours, add dropwise complete. The reaction was incubated at 95°C for 2 hours, poured into ice, filtered, and dried to obtain 399.6 g (93% yield) of the title compound, with a content greater than 95%.
Embodiment 3
[0034] Preparation of 1,5-difluoro-2,4-dinitrobenzene:
[0035]Add 2,4-difluoronitrobenzene (320 g, 2 mol) to a 2000 mL reaction flask, add 600 mL of concentrated sulfuric acid dropwise at room temperature, and complete the dropwise addition within 2 hours, start adding 250 mL of fuming nitric acid dropwise, and complete the dropwise addition within 4 hours. The reaction was carried out at 100°C for 2 hours, poured into ice, filtered and dried to obtain 399.3 g (90% yield) of the title compound with a content of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com