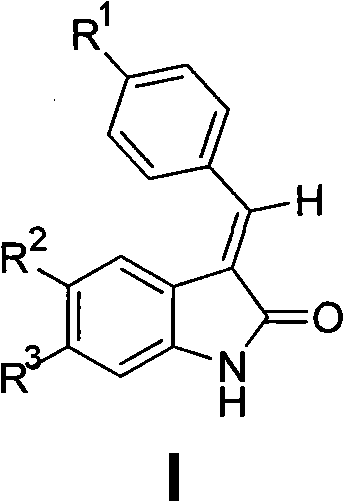
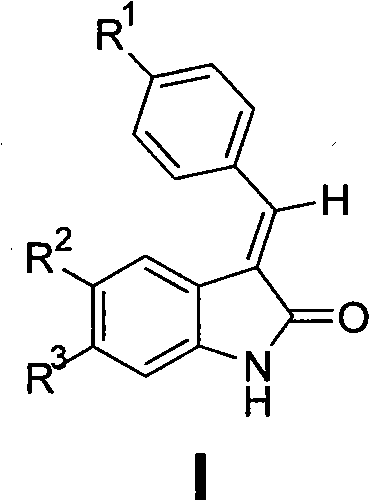
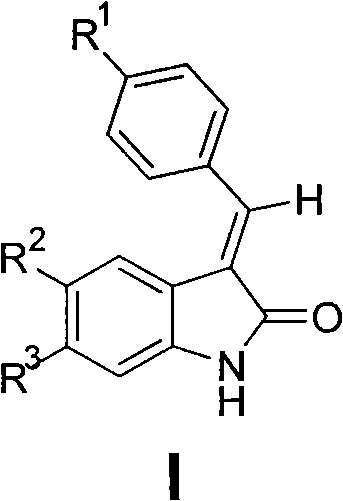
2-indolone compound with anti-inflammatory activity, preparation method and medicinal application thereof
A compound, indolinone technology, applied in the field of new 2-indolinone compounds and their preparation, can solve the problems of bronchial asthma and spasm, aggravate the development of inflammation, increase the generation of LTs, etc., and achieve the effect of good analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Preparation of E-3-{[4-(sulfamoyl)phenyl]methylene}-2-indolinone (101)
[0101]
[0102] Add p-sulfamoylbenzaldehyde (2mmol), 2-indolinone (2mmol), molten sodium acetate (7mmol) and 5mL glacial acetic acid into the reaction flask, heat to reflux, and track the reaction by TLC until the raw material spots disappear. Cool to room temperature, filter with suction, wash with water, dry, and recrystallize from ethyl acetate to obtain orange-yellow crystals, yield 45%, mp 230-232°C. IR (KBr, cm -1 ): 3357 (NH 2 ), 3263 (NH 2 ), 1687 (C=O), 1334 (SO 2 ), 1163 (SO 2 ). 1 H NMR (300MHz, DMSO-d 6 )δ: 6.84-6.90 (m, 2H, ArH), 7.24 (t, 1H, J = 7.2Hz, ArH), 7.43 (d, 1H, J = 7.2Hz, ArH), 7.47 (s, 2H, NH 2 ), 7.64(s, 1H, =CH), 7.87(d, 2H, J=8.1Hz, ArH), 7.94(d, 2H, J=8.1Hz, ArH), 10.67(s, 1H, NH).ESI -MS (m / z): 299[M-H] - .Anal.(C 15 h 12 N 2 o 3 S, C%, H%, N%): 55.99, 4.03, 9.33; Found: 56.13, 4.08, 9.24.
Embodiment 2
[0104] Preparation of E-3-{[4-(sulfamoyl)phenyl]methylene}-5-fluoro-2-indolinone (102)
[0105]
[0106] Referring to the preparation method of 101, by reacting p-sulfamoylbenzaldehyde and 5-fluoro-2-indolinone, recrystallized from ethyl acetate, an orange-yellow powdery solid was obtained, yield 62.9%, mp 196~198°C. IR (KBr, cm -1 ): 3353 (NH 2 ), 3242 (NH 2 ), 1695 (C=O), 1317 (SO 2 ), 1157 (SO 2 ). 1 H NMR (300MHz, DMSO-d 6 )δ: 6.88(s, 1H, ArH), 7.10-7.13(m, 2H, ArH), 7.47(s, 2H, NH 2 ), 7.72(s, 1H, =CH), 7.88(d, 2H, J=8.4Hz, ArH), 7.96(d, 2H, J=8.4Hz, ArH), 10.69(s, 1H, NH).ESI - MS (m / z): 317 [M-H] - .Anal.(C 15 h 11 FN 2 o 3 S, C%, H%, N%): 56.60, 3.48, 8.80; Found: 56.36, 3.35, 8.95.
Embodiment 3
[0108] Preparation of E-3-{[4-(sulfamoyl)phenyl]methylene}-5-chloro-2-indolinone (103)
[0109]
[0110] Referring to the preparation method of 101, react p-sulfamoylbenzaldehyde and 5-chloro-2-indolinone, and recrystallize from ethyl acetate to obtain an orange-red solid with a yield of 46.3%, mp 216-218°C. IR (KBr, cm -1 ): 3342 (NH 2 ), 3249 (NH 2 ), 1718 (C=O), 1301 (SO 2 ), 1157 (SO 2 ). 1 H NMR (300MHz, DMSO-d 6 )δ: 6.91(d, 1H, J=8.1Hz, ArH), 7.31(d, 1H, J=8.1Hz, ArH), 7.34(s, 1H, ArH), 7.52(s, 2H, NH 2 ), 7.73(s, 1H, =CH), 7.89(d, 2H, J=8.1Hz, ArH), 7.97(d, 2H, J=8.1Hz, ArH), 10.82(s, 1H, NH).ESI -MS (m / z): 333[M-H] - .Anal.(C 15 h11 ClN 2 o 3 S, C%, H%, N%): 53.82, 3.31, 8.37; Found: 54.11, 3.24, 8.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com