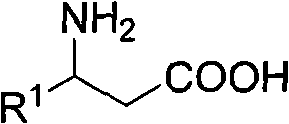
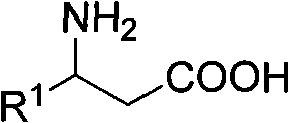
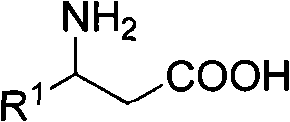
3-amino-3-arylpropionic acid and preparation method thereof
A technology of aryl propionic acid and amino, applied in the field of 3-amino-3-aryl propionic acid and its preparation, achieving the effects of simple method, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 3-amino-3-phenylpropionic acid
[0022]
[0023] 3.18g of benzaldehyde (30mmol), 3.14g of malonic acid (30mmol) and 4.63g of ammonium acetate (60mmol) were refluxed in 60mL of methanol for 6 hours; after cooling to room temperature, filtered to obtain 3.67g of 3-amino-3-phenyl Propionic acid, yield 74%.
[0024] H NMR spectrum ( 1 H NMR, 300MHz, DMSO) δ: 12.20(s, 1H), 7.29-7.45(m, 5H), 5.15(s, 2H), 4.90(m, 1H), 2.87(dd, J=6.6, 1.8Hz, 1H), 2.62 (dd, J=6.6, 1.8 Hz, 1H). Mass spectrum (ESI): 166 (M+H + , 100), 188 (M+Na + , 42.5), 204 (M+K + , 15.3).
Embodiment 2
[0025] Example 2 Synthesis of 3-amino-3-(4-fluorophenyl)propionic acid
[0026]
[0027] Reflux 62g 4-fluorobenzaldehyde (0.5mol), 52g malonic acid (0.5mol) and 77g ammonium acetate (1mol) in 500mL ethanol for 8 hours; after cooling to room temperature, filter to obtain 73.3g 3-amino-3 - Phenylpropionic acid, yield 80%.
[0028] H NMR spectrum ( 1 H NMR, 300MHz, DMSO) δ: 12.18(s, 1H), 7.27(d, J=7.5Hz, 2H), 7.19(d, J=7.5Hz, 2H), 5.18(s, 2H), 4.92(m , 1H), 2.88 (dd, J=6.6, 1.8Hz, 1H), 2.65 (dd, J=6.6, 1.8Hz, 1H). Mass Spectrum (ESI): 184 (M+H + , 100), 206 (M+Na + , 32.8), 223 (M+K + , 12.6).
Embodiment 3
[0029] Example 3 Synthesis of 3-amino-3-(3-nitro-4-fluorophenyl)propionic acid
[0030]
[0031] Reflux 14.5g 3-nitro-4-fluorobenzaldehyde (0.087mol), 9.0g malonic acid (0.087mol) and 13.4g ammonium acetate (0.174mol) in ethanol for 6 hours; after cooling to room temperature, filter, 17.5 g of 3-amino-3-(3-nitro-4-fluorophenyl)propionic acid (0.076 mol) were obtained, yield 88%.
[0032] H NMR spectrum ( 1 H NMR, 300MHz, DMSO) δ: 12.22(s, 1H), 8.18(s, 1H), 7.67(d, J=7.5Hz, 1H), 7.48(d, J=7.5Hz, 1H), 4.94(m , 1H), 2.88 (dd, J=6.6, 1.8Hz, 1H), 2.64 (dd, J=6.6, 1.8Hz, 1H). Mass spectrum (ESI): 329 (M+H + , 100), 351 (M+Na + , 23.5), 367 (M+K + , 7.2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com