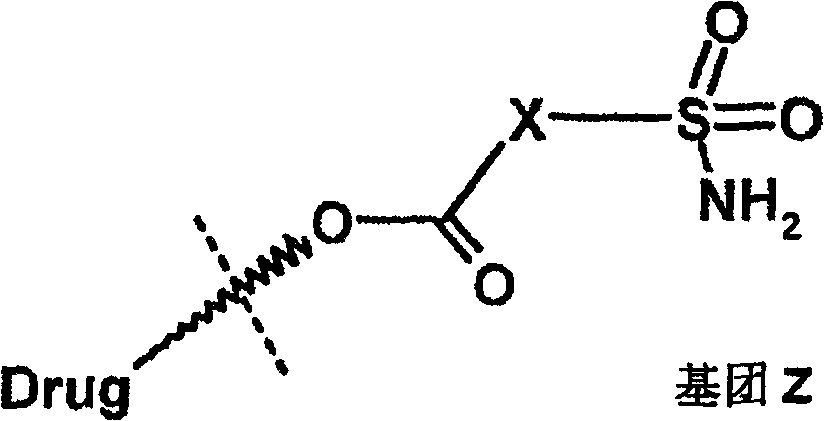
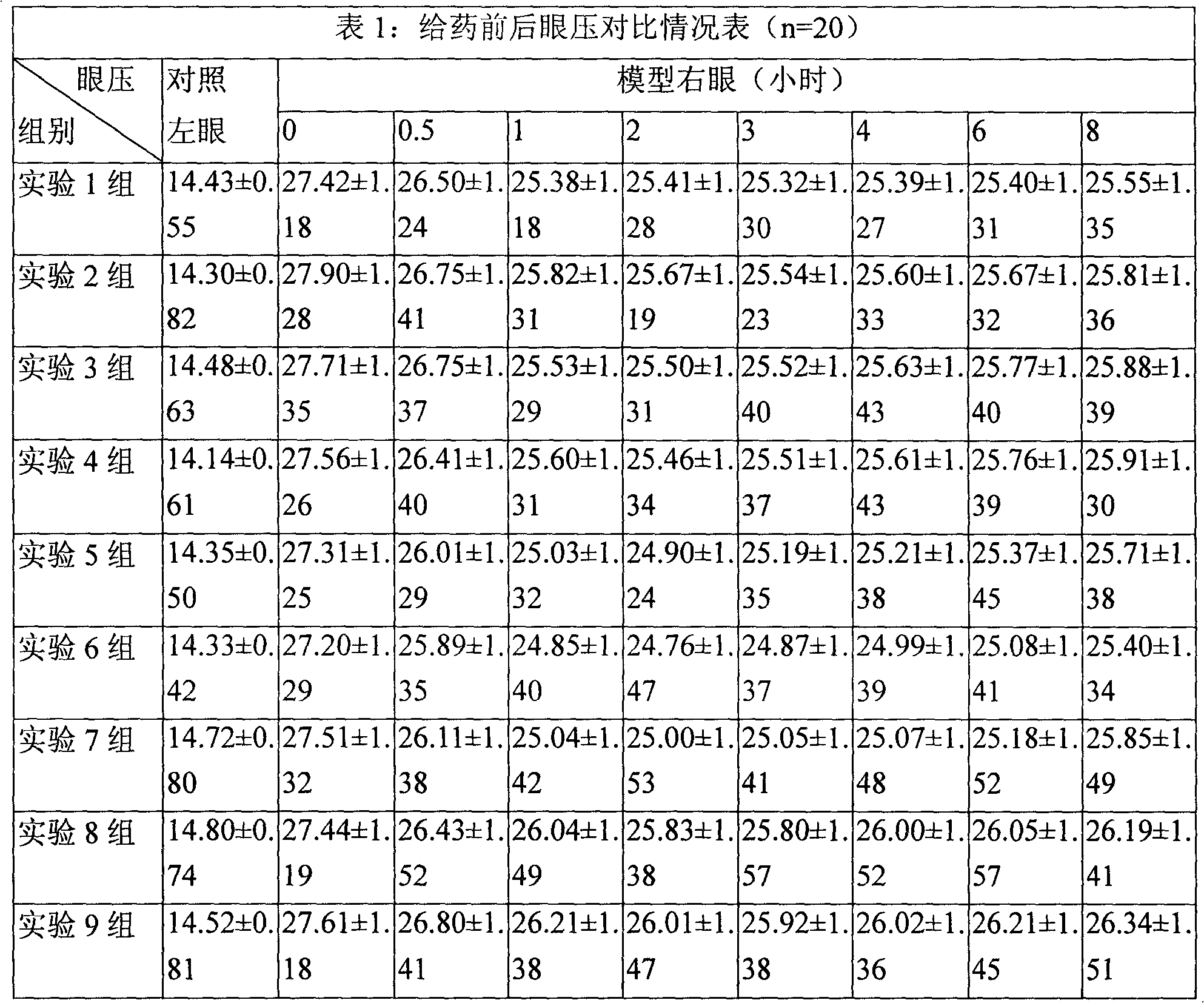
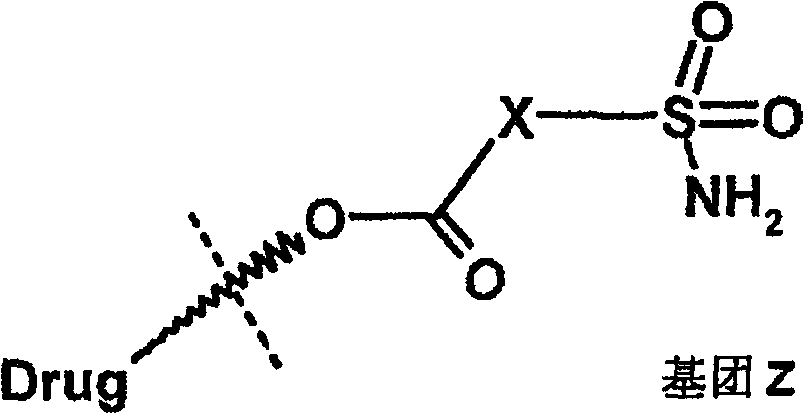
Ophthalmic composition of heteroaryl sulfamoyl carboxylic ester carbonic anhydrase inhibitor
A technology of sulfamoyl nicotinic acid ester and heteroaromatic group, which is applied in the field of solution preparations containing cyclodextrin, and can solve the problems such as the same effect cannot be explained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis Example 1: Mometasone-17-6'-sulfamoyl nicotinate
[0076] Put 1 mmol of mometasone in 30 ml of pyridine under nitrogen, add 1.3 mmol of 6-aminosulfonyl nicotinic acid at 0 degrees, add DMAP0.1 mmol and stir for 3 hours, stir the reaction mixture into 300 ml of water and use Acidify with 10% HCl. After 10 minutes, it was filtered off with suction, washed with water and dried. The residue was chromatographically purified on silica gel to obtain 0.47 mmol of mometasone-17-6'-sulfamoyl nicotinate.
Embodiment 2
[0077] Synthesis Example 2: Prednisolone-21-2'-ethyl-5'-sulfamoylthiophene-3'-carboxylate
[0078] 1.3 mmol of 5-(sulfamoyl)-2-ethyl-3-thiophenecarboxylic acid were dissolved in 50 ml of pyridine under argon. Add 1mmol of prednisolone at -5°C, add PTS0.05mmol and DCC0.05mmol and stir for 8 hours, stir and dilute the reaction mixture in 500ml of water, adjust the pH to 6 with hydrochloric acid. After 10 minutes, suction filter, wash with water and dry. The residue was purified by chromatography on silica gel to obtain 0.62 mmol of prednisolone-21-2'-ethyl-5'-sulfamoylthiophene-3'-carboxylate.
[0079] Hydrocortisone-17-butyrate-21-6'-sulfamoyl nicotinate, fluorometholone-17-6'-sulfamoyl nicotinate, dexamethasone-21-5'-sulfamoyl nicotinate Acyl-thiophene-3'-carboxylates can also be prepared analogously.
Embodiment 1-1
[0081] 3-Hydroxyestro-1,3,5(10)-trien-17β-yl-6’-sulfamoylnicotinate (particle size 5-20μm) 1.0g
[0082] Accessories:
[0083] Sodium Carboxymethyl Cellulose 2.0g
[0084] Tween-80 0.8g
[0085] Sodium dihydrogen phosphate amount
[0086] Disodium hydrogen phosphate appropriate amount
[0087] Sodium chloride appropriate amount
[0088] Ethylparaben 0.1g
[0089] Propylparaben 0.1g
[0090] Water for injection 1000ml
[0091] Dissolve the prescribed amount of ethylparaben and propylparaben in water for injection with 50% of the prescribed amount, heat to 80-90°C, add the prescribed amount of Tween-80 to dissolve, and use No. 3 vertical melting Filter through a funnel and use as solution. Dissolve sodium carboxymethyl cellulose in water for injection with 30% of the prescription volume, filter with a Buchner funnel with 200-mesh nylon cloth, heat to 80-90°C, and add to the prescription Amount of 3-hydroxyestra-1,3,5(10)-trien-17β-yl-6'-sulfamoyl nicotinate was stirred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com