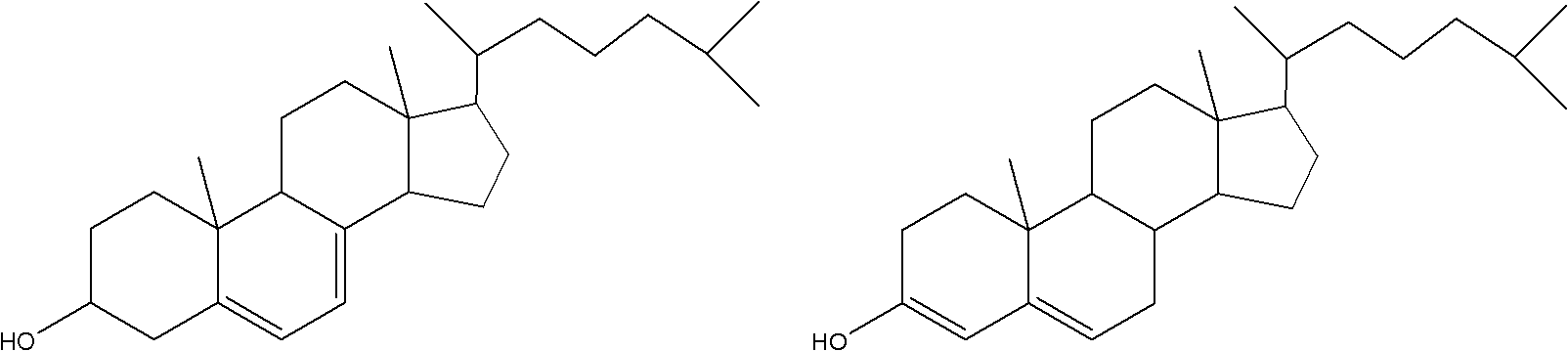
Method for purifying 7-dehydrocholesterol leftovers
A technology for dehydrocholesterol and a purification method, which is applied in the field of purification of intermediates, can solve the problems of 7-dehydrocholesterol loss, complicated process and high cost, and achieves the effects of high industrial feasibility, simple reaction process and reduced processing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Add 500 ml of methanol into 100.5 g of 7-dehydrocholesterol scrap containing 10.2 g of 7-dehydrocholesterol, heat up to 50° C. for dissolution, and then add Al 2 (SO 4 ) 3 5.1g, after 1 hour of reaction, no 3-hydroxycholesta-4,6-diene was detected, then 200ml of distilled water and 300ml of n-hexane were added and extracted three times, then the silica gel chromatography column was used to obtain the content of 7-dehydrocholesterol 25.8 g of a mixture with a content of 34.5%, and 10.3 g of a mixture with a 7-dehydrocholesterol content of 68.5% were obtained after recrystallization from 10 times the volume of methanol.
Embodiment 2
[0020] Example 2: Add 500 ml of ethanol to 103.2 g of 7-dehydrocholesterol offal containing 11.2 g of 7-dehydrocholesterol, heat up to 35° C. to dissolve, and then add AlCl 3 6.2g, after 35 minutes of reaction, no 3-hydroxycholesta-4,6-diene was detected, then 200ml of distilled water and 300ml of n-hexane were added and extracted three times, then applied to a silica gel chromatography column to obtain a 7-dehydrocholesterol content of 37.6 % of the mixture 26.5g, after 8 times the volume of ethanol recrystallization to obtain 7-dehydrocholesterol content of 72.5% of the mixture 11.5g.
Embodiment 3
[0021] Example 3: 105.5 g of 7-dehydrocholesterol residue containing 10.5 g of 7-dehydrocholesterol was put into 500 ml of ethanol, and after being heated to 45° C. for dissolution, FeCl was put into 3 5.5g, after 50 minutes of reaction, no 3-hydroxycholesta-4,6-diene was detected, then 200ml of distilled water was added, 300ml of n-hexane was fully extracted three times and then applied to a silica gel chromatography column to obtain a 7-dehydrocholesterol content of 35.5 % of the mixture 25.9g, 10.8g of the mixture with a 7-dehydrocholesterol content of 75.2% was obtained after recrystallization from 10 times the volume of methanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com