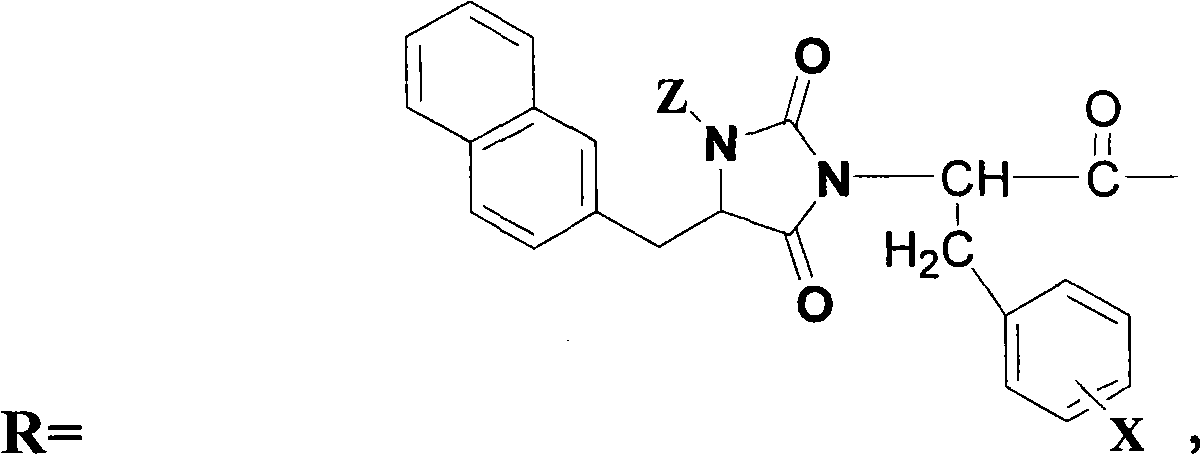
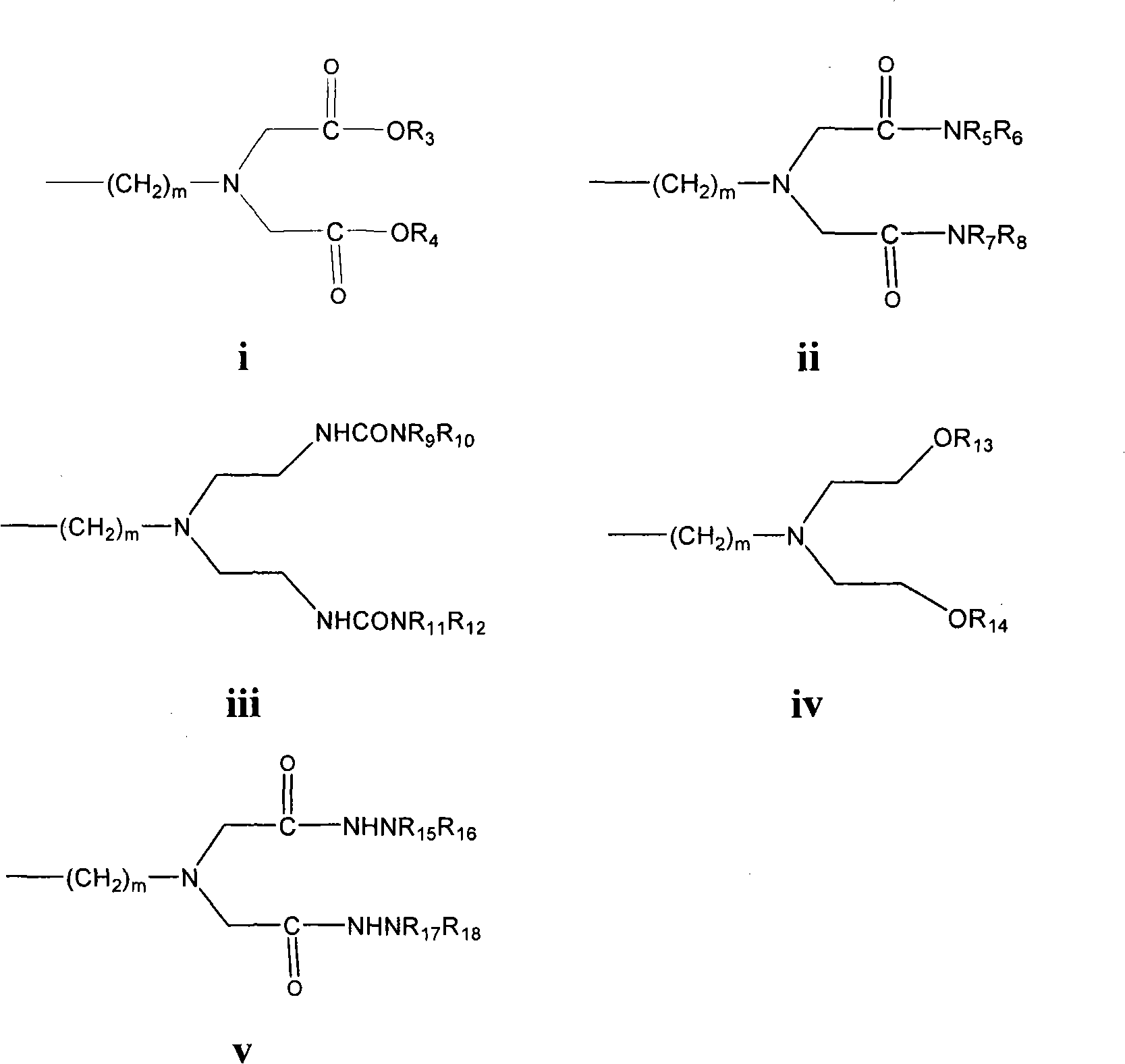
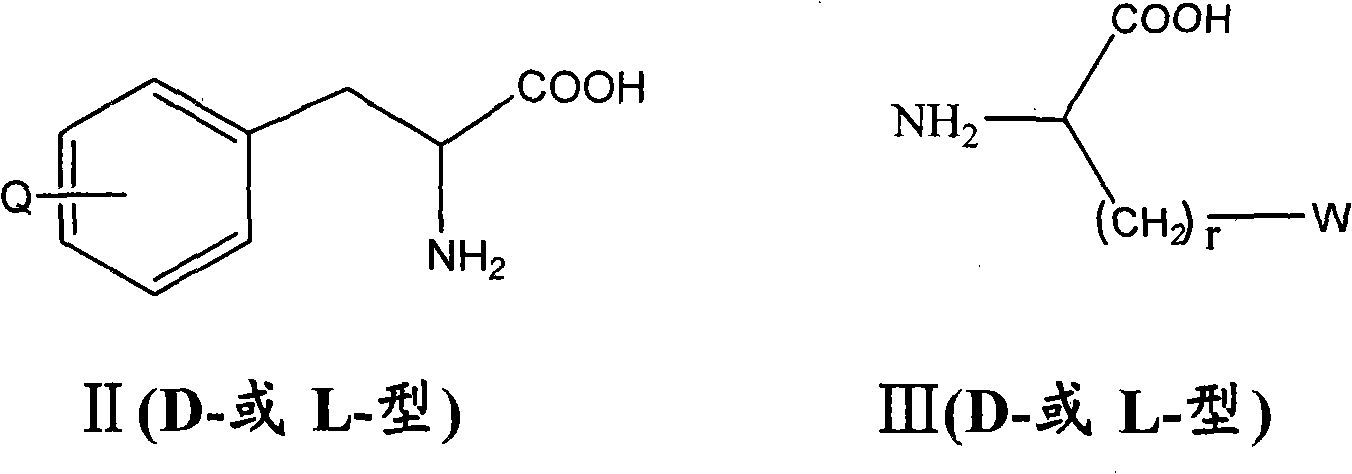
Antagonist of luteinizing hormone releasing hormone (LHRH) containing hydantoin structure
A technology of stereoisomers and configurations, applied in the field of decapeptide derivatives, can solve the problems of limited application of LHRH antagonists, low bioavailability, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0305] Embodiment 1: the synthesis of compound 1
[0306] NHC-D-Phe 3 -Ser 4 -Mop 5 -D-Aph 6 (Diethyl-Cbm)-Leu 7 -Arg 8 -Pro 9 -D-Ala 10 -NH 2
[0307] With 63mg MBHA resin (0.04mmol) as the solid-phase carrier, BOP / DIEA as the condensing agent, according to the amino acid sequence of the compound, according to the standard Boc solid-phase peptide synthesis method (references: Huang Weide, Chen Changqing, Polypeptide Synthesis, Science Press , 1985) operation synthesis Boc-D-Nal 1 -D-Cpa 2 -D-Phe 3 -Ser 4 -Mop 5 -D-Aph 6 (Diethyl-Cbm)-Leu7 -Arg 8 -Pro 9 -D-Ala 10 -NH 2 - MBHA resin.
[0308] After Boc-D-Nal is connected to the peptide chain, use 4M HCl / Diox to remove the Boc at the N-terminus, neutralize, after washing, add 3 times excess CDI, react for 0.5h, wash off excess CDI with DCM, indene Test the condensation effect of triketone, if it is positive, react again until it is negative, add 10% DIEA / DCM, react at room temperature for 4 hours, wash the r...
Embodiment 2~48
[0310] Embodiment 2~48: the synthesis of compound 2~48
[0311] Compounds 2-48 were synthesized using a method similar to Example 1. The structures of compounds 2-48 are respectively referred to above. Various raw materials used in the synthesis of compounds 2-48 were purchased from Chengdu Chengnuo Technology Co., Ltd. and Nankai Synthetic Technology Co., Ltd., or synthesized by the inventors themselves.
[0312] The synthesis results are summarized in Table 1 below:
[0313] Table 1
[0314] Compound No.
[0315] 31~50
[0316] * "ESI-MS difference" means the absolute value of the difference between the theoretical value of ESI-MS and the measured value, ie: ESI-MS difference = |theoretical value-measured value|.
experiment example 1
[0317] Experimental Example 1: In vivo testosterone inhibition experiment in rats:
[0318] Before the experiment, the weight of the animals was weighed, and the blood was collected from the venous plexus behind the glass capillary bulb. After the serum was separated, the serum testosterone content was measured by chemiluminescence method. Randomly grouped according to the testosterone content and body weight, 4 animals in each group were injected subcutaneously with the compound to be tested in a one-time overdose. 1, 2, 4, 6, 8, 10, 12, 16, 20, and 24 hours after 500 μg, blood was collected from the retrobulbar venous plexus, centrifuged at 5000 rpm for 8 min, and the separated serum was analyzed by chemiluminescence (Access Immunoassay System, Beckman Coulter, USA). Chemiluminescence instrument) was used to measure serum testosterone levels. The results are shown in Table 2.
[0319] Table 2
[0320] Compound number a
Inhibition of testosterone action tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com